In case of PCl5 lone pair electrons are present
A
s-orbital
B
p-orbital
C
sp3-o-orbital
D
sp2-orbital
Text Solution
The correct Answer is:2 or 3
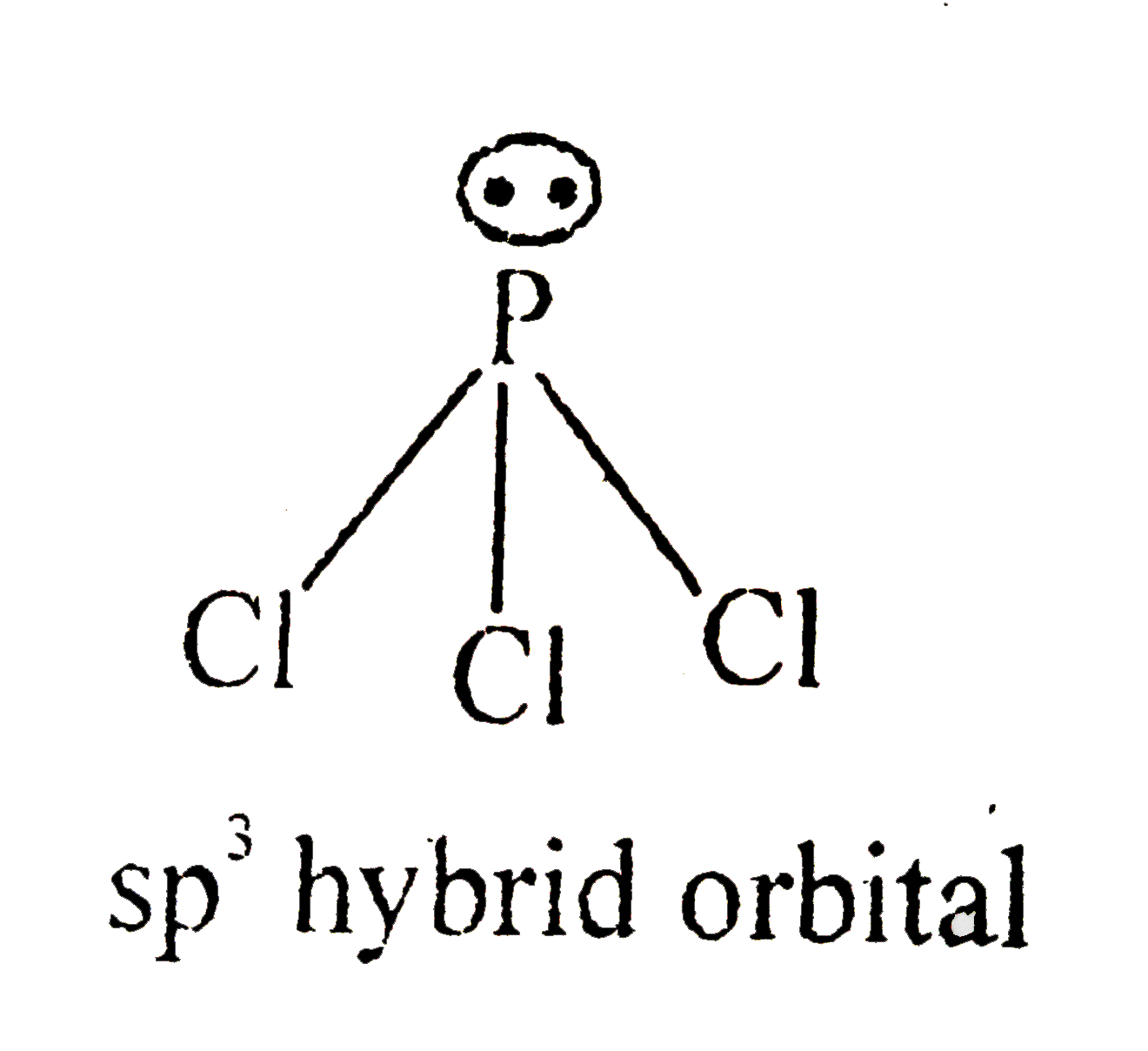
sp3 hybrid orbital
|
Similar Questions
Knowledge Check
In ClF3, lone pair of electrons is present at equatorial position to minimize :
ALone pair –lone pair repulsion onlyBLone pair-bond pair repulsion mainlyCLone pair –lone pair and lone pair-bond pair repulsion onlyDLone pair-lone pair and bond pair-bond pair repulsion onlyIn the Lewis structure for formic acid, HCOOH, how many bonding pairs and lone pairs of electrons are present?
A4 bonding, 2 loneB4 bonding, 5 loneC5 bonding, 0 loneD5 bonding, 4 loneHow many lone pair of electrons are present on chlorine in ClF3 molecule ?
A0B1C2D3
In a singlet carbene, the lone pair of electrons is present in a ..........hybridised orbital.
View SolutionThe total number of lone pairs of electrons present in Cl2O7 molecule is
View SolutionIn the following compounds of xenon, highest number of lone pairs of electrons is present in :
View SolutionTwo lone pairs of electron and two bond pair of
electrons are presnet in
View SolutionWhich has maximum number of lone pair of electrons present on central atom i.e., xenon?
View Solution

