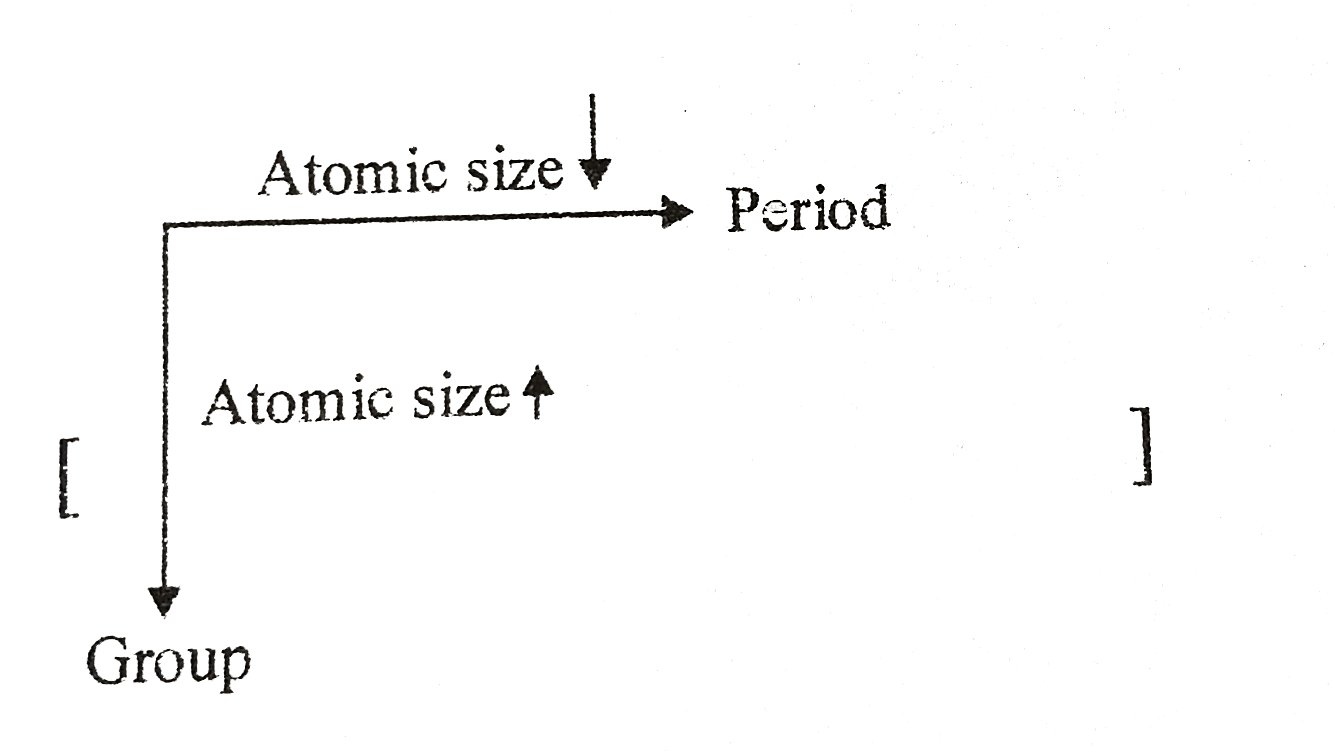Arrange in decreasing order of atomic size: Na,Cs,Mg,Si,CI
Video Solution
Text Solution
The correct Answer is:[Cs>Na>Mg>Si>CI]
|
Similar Questions
Knowledge Check
Arrange the following in increasing order of their atomic radius: Na, K, Mg, Rb
AMg<K<Na<RbBMg<Na<K<RbCMg<Na<Rb<KDNa<K<Rb<MgThe correct arrangement of decreasing order of atomic radius among Na,K,Mg and Rb is
ARb>Na>N>MgBK>Rb>Na>MgCRb>K>Na>MgDMg>Rb>K>NaArrange the following in increasing order of their atomic radius: Na, K, Mg, Rb
AMg<K<Na<RbBMg<Na<K<RbCMg<Na<Rb<KDNa<K<Rb<Mg
Arrange the following in decreasing atomic size :
(i) Na, Mg, K (ii) N, F, O (iii) N, S, P
View SolutionArrange the following ions in the increasing order of atomic size.
Na+,Mg2+,Al3+
View SolutionThe correct arrangement of increasing order of atomic radius among Na, K, Mg, Rb is
View SolutionArrange marked atom in decreasing order of acidic strength
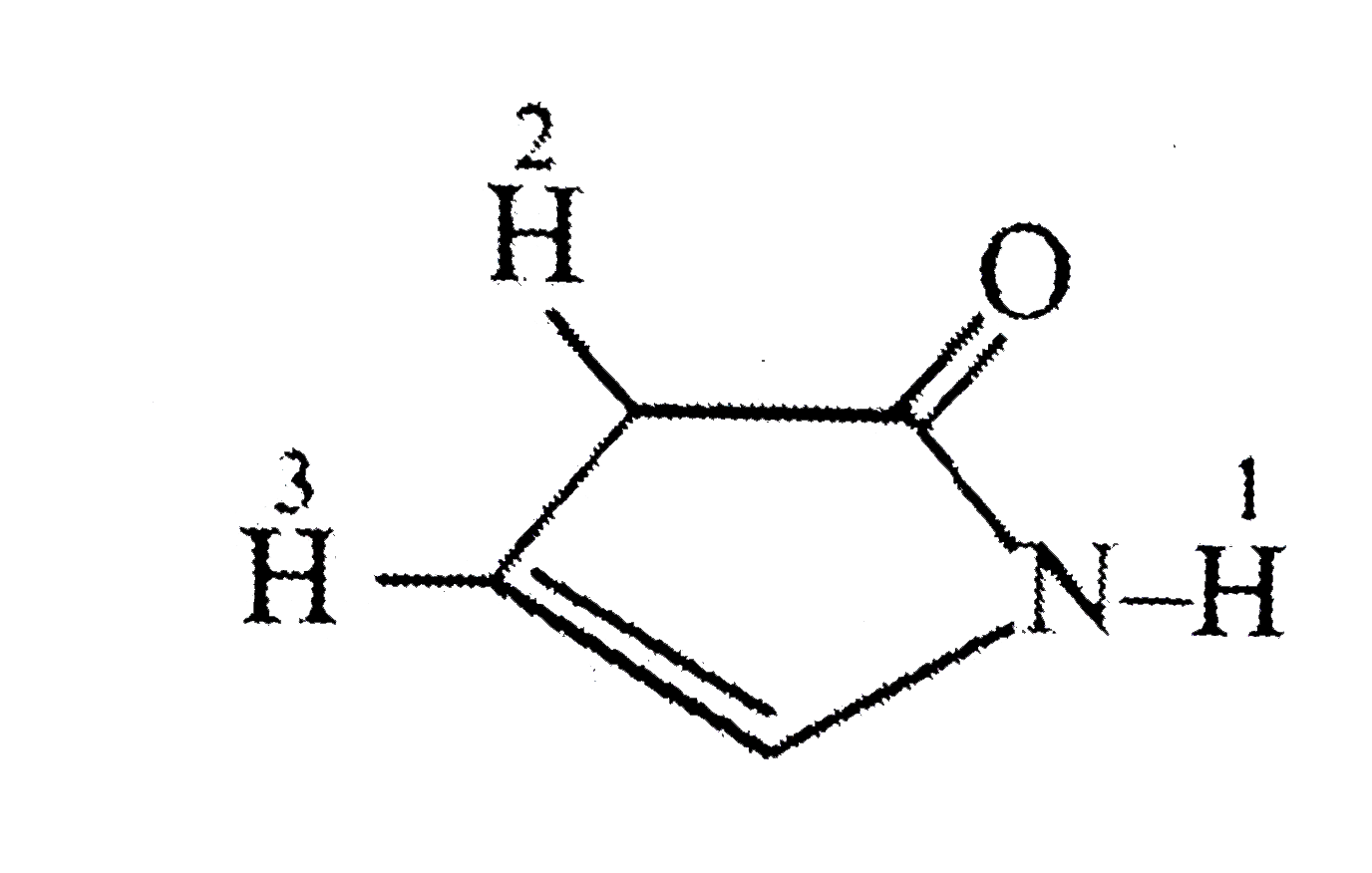
View SolutionThe increasing order of the atomic radius of Si,S,Na,Mg,Al is
View Solution