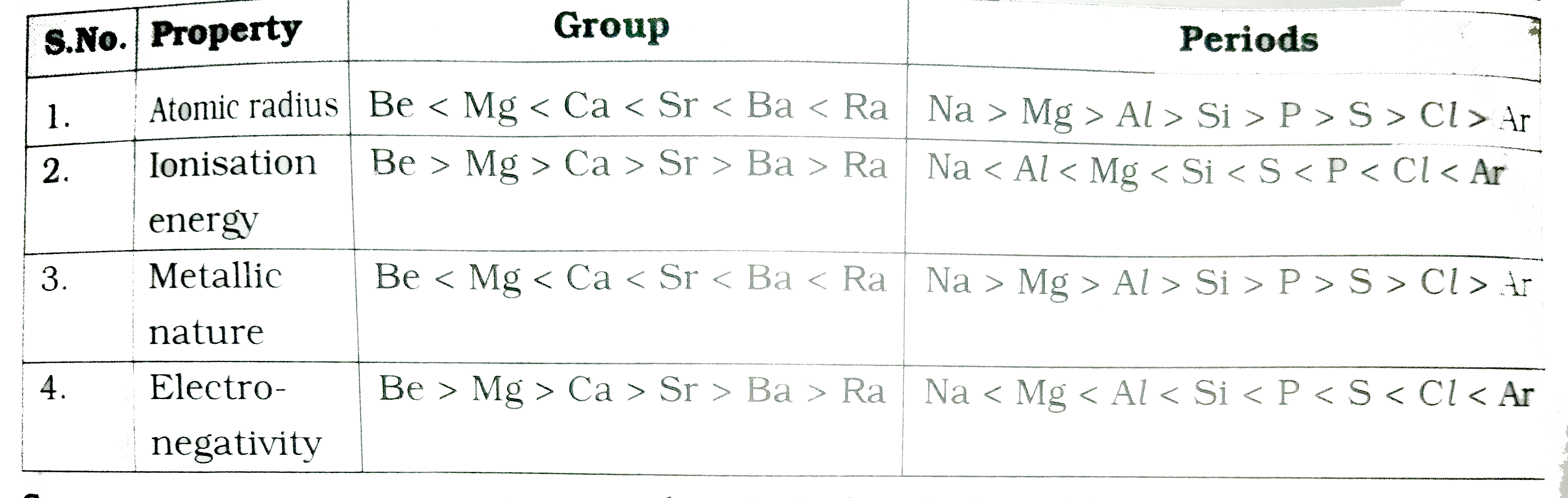Group : Be - Mg -Ca -Sr -Ba -Ra
Period : Na -Mg -Al - Si - S -Ci -Ar
Write the order of increasing or decreasing in the given boxes .
 Solution in Telugu
Solution in Telugu
Video Solution
Text Solution
|
Similar Questions
Arrange the following members in increasing order of size Be, Mg, Ca, Ba, Sr
View SolutionThe elements Na, Mg, Al, Si, P, S, Cl and Ar belong to the second period of the Periodic Table.
View SolutionArrange the following elements in the order of their decreasing metallic character: Na, Si, CI, Mg, Al.
View SolutionGive orbital picture of following elements Na Mg Al ,Si P , S , CI , Ar , K , Ca
View SolutionNa, Mg, Al एव Si तत्वों की प्रथम आयनीकरण एन्थेलपी का बढ़ता क्रम है
View SolutionThe lattice energies of the oxides of Mg, Ca, Sr and Ba follow the order
View SolutionArrange Mg,Al,P,Si,S in decreasing order of ionization enthalpy
View SolutionBa, Sr, Ca and Mg may be arranged in order of decreasing first ionisation energy (IE_1) as:
View Solution