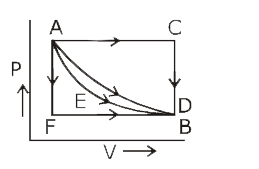
An ideal system can be brought from stage A to B through Four paths as shown in the figure. The energy given to the system is minimum is: 
A
path ACB
B
path ADB
C
path AEB
D
path AFB
Text Solution
The correct Answer is:D
△u= some for all
work is minimum for AFB
⇒△QAFB is minimum
|
Similar Questions
Knowledge Check
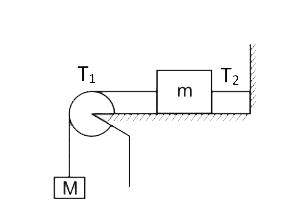
For the system shown in figure, the acceleration of the block is :-
 A1m/s2B2m/s2C3m/s2D4m/s2
A1m/s2B2m/s2C3m/s2D4m/s2A gas of given mass, is brought from stage A to B along three paths 1, 2 and 3, as shown in the figure. If the amount of work done in these three processes is respectively equal to W1,W2 and W3, then–
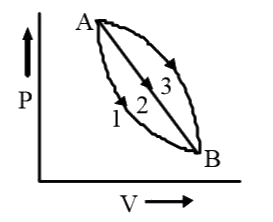 AW1>W2>W3BW1<W2<W3CW1=W2=W3DW1<W2,W3<W2
AW1>W2>W3BW1<W2<W3CW1=W2=W3DW1<W2,W3<W2One mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in the figure. The change in internal energy of the gas during the transition is
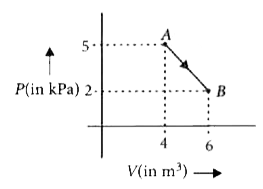 A20 JB−12kJC20 kJD−20kJ
A20 JB−12kJC20 kJD−20kJ
For the given situation as shown in the figure, the value of q θ keep the system in equilibrium will be

View Solution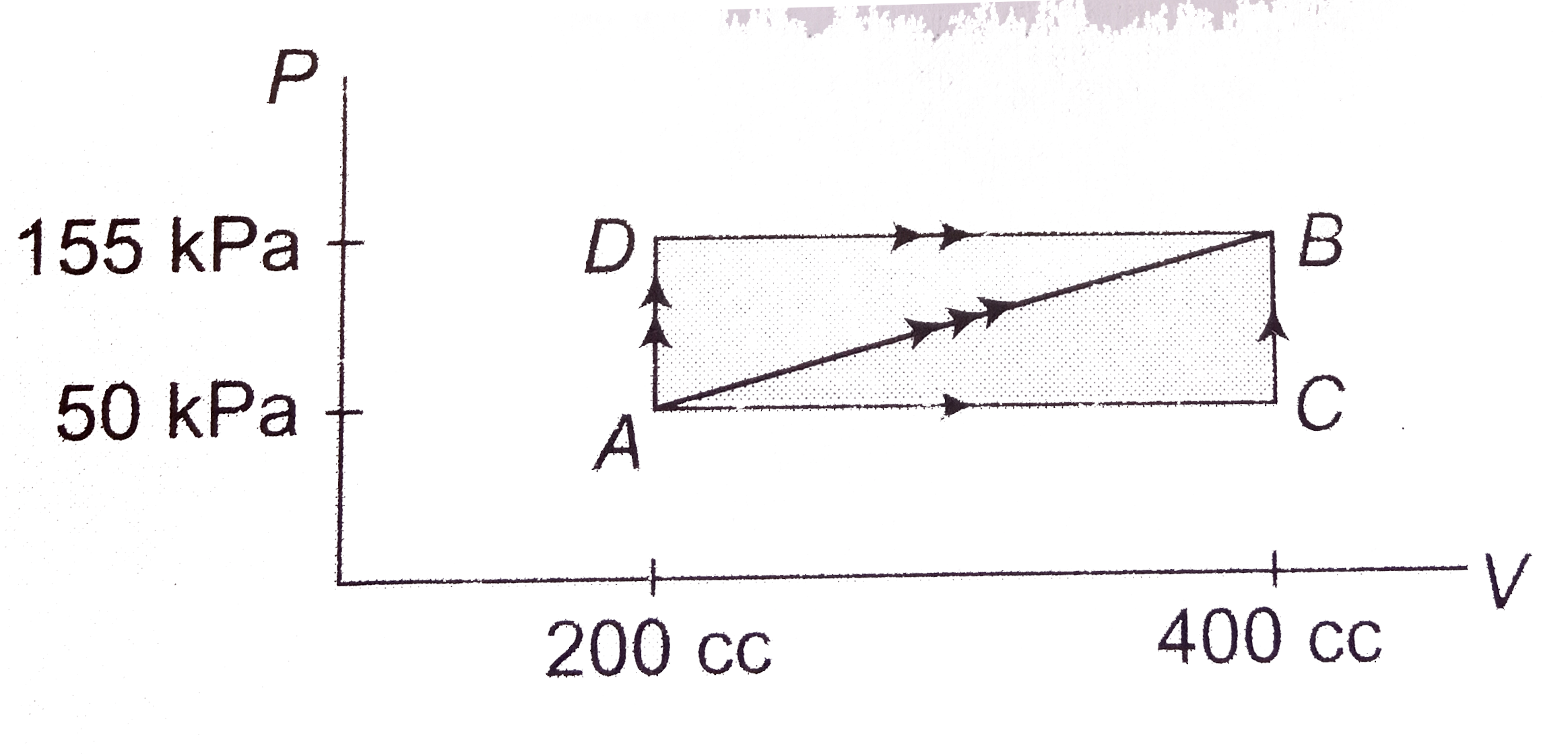
50 cal of heat should be supplied to take a system from the state A to the state B through the path ACB as shown in Figure. The quantity of heat to be supplied to take it from A to B via ADB.
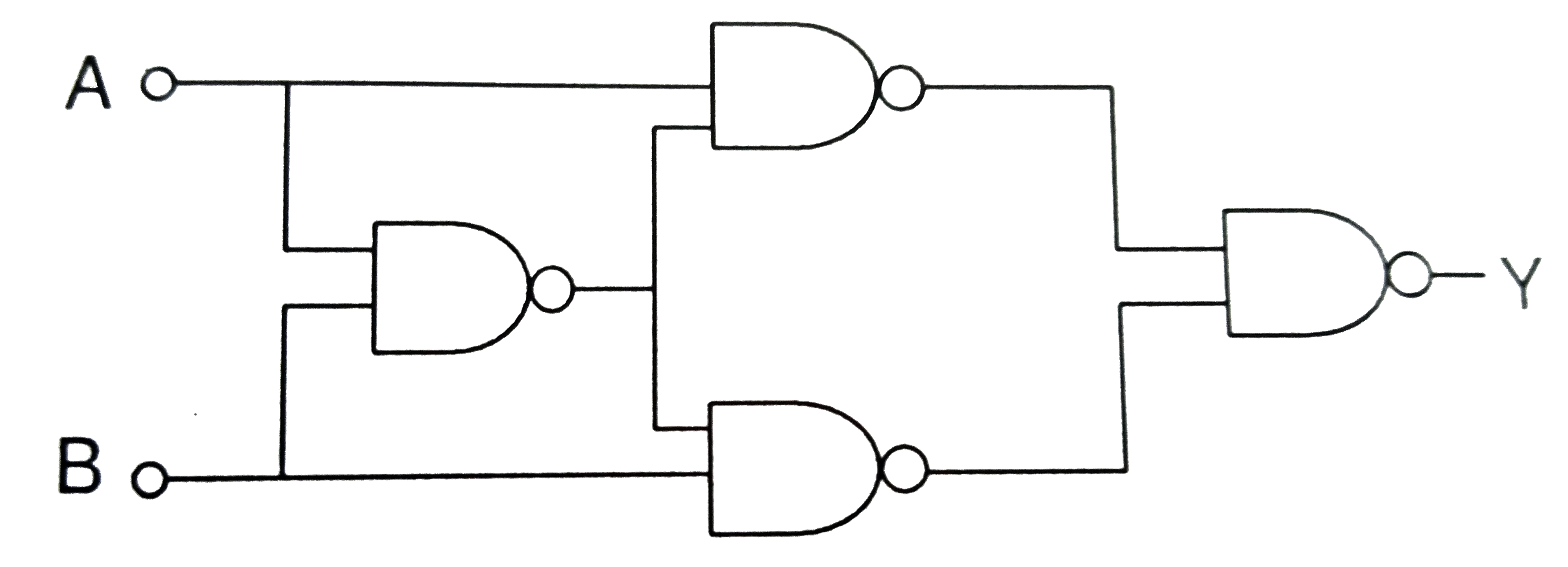
View SolutionTruth table for system of four NAND gates as shown in figure is
View SolutionCorrect path of energy flow in a system is
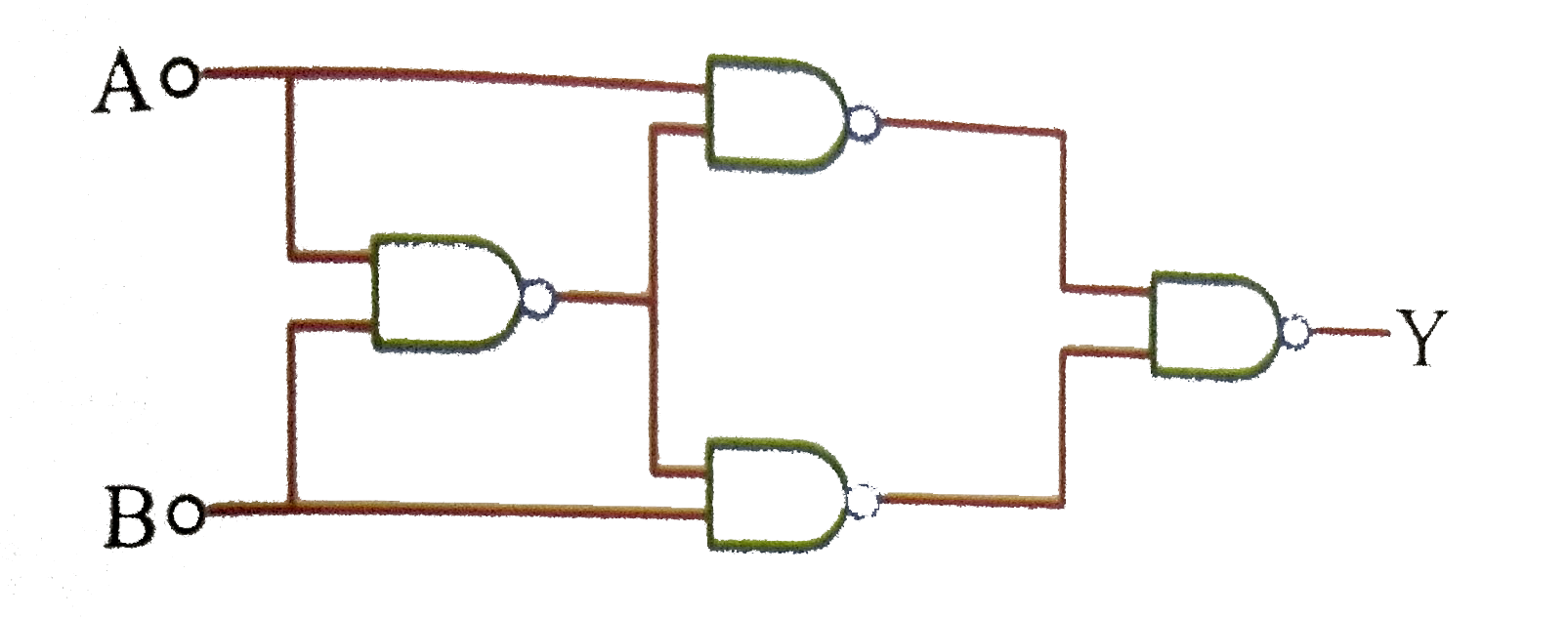
View SolutionTruth table for system of four NAND gates as shown in figure is :
 .
.
View Solution