An ideal system can be brought from stage A to B through four paths as shown in the figure. The heat energy given to the system is minimum in :
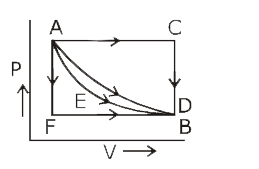
path ACB
path ADB
path AEB
path AFB
The correct Answer is:D
Step by step video & image solution for An ideal system can be brought from stage A to B through four paths as shown in the figure. The heat energy given to the system is minimum in : by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
An ideal system can be brought from stage A to B through Four paths as shown in the figure. The energy given to the system is minimum is:
 Apath ACBBpath ADBCpath AEBDpath AFB
Apath ACBBpath ADBCpath AEBDpath AFBA gas of given mass, is brought from stage A to B along three paths 1, 2 and 3, as shown in the figure. If the amount of work done in these three processes is respectively equal to W1,W2 and W3, then–
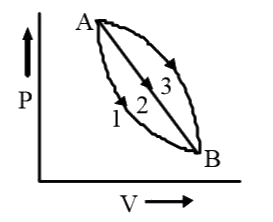 AW1>W2>W3BW1<W2<W3CW1=W2=W3DW1<W2,W3<W2
AW1>W2>W3BW1<W2<W3CW1=W2=W3DW1<W2,W3<W2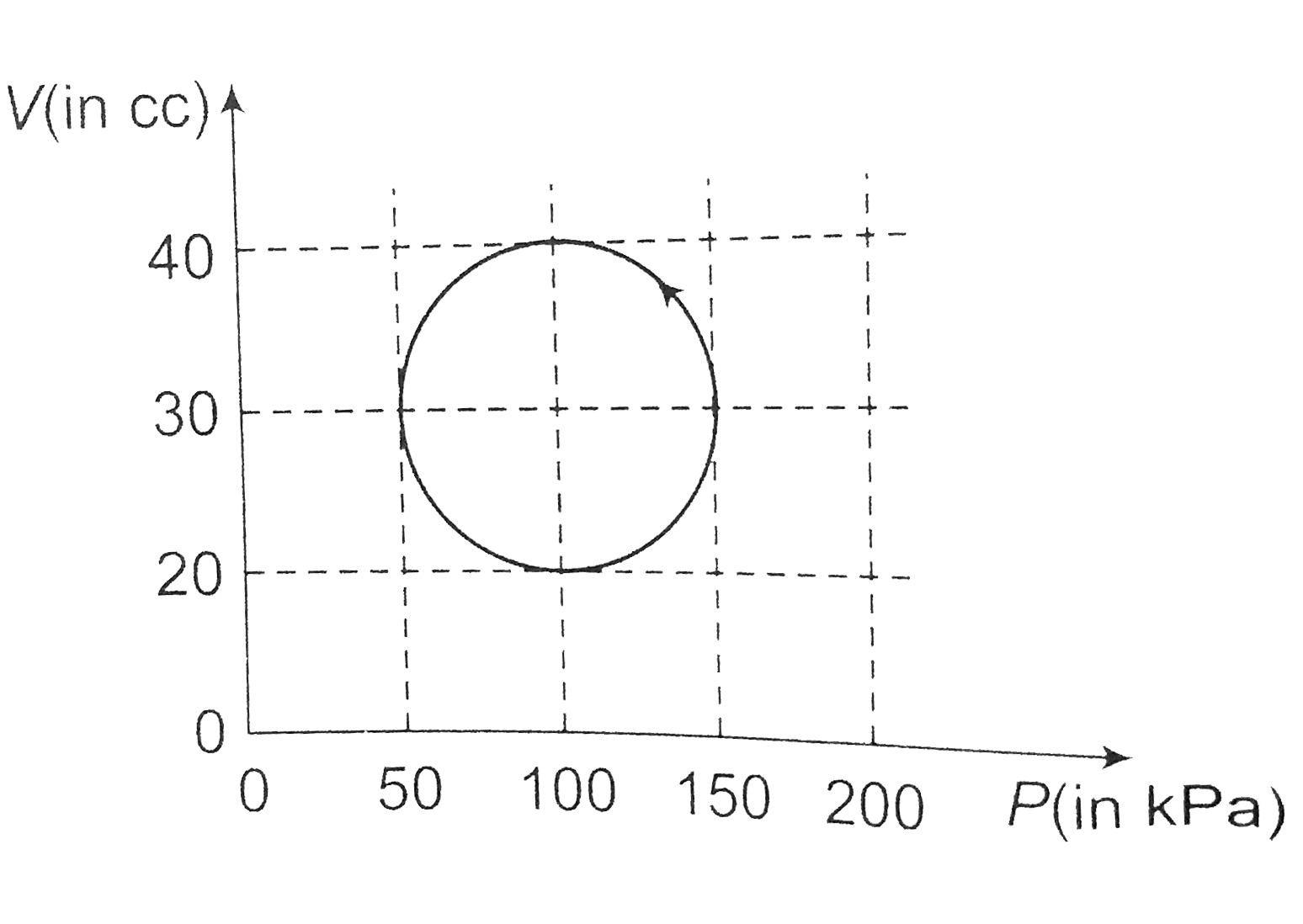
A system is taken through a cyclic process represented by a circle as shown in the figure. The heat absorbed by the system isAπ×103JBπ2JC4π×102JDπJ
A system of three charges are placed as shown in the figure :
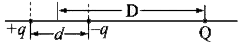
If D>>d, the potential energy of the system is :
View SolutionWhen a system is taken from state A to state B along path ACB as shown in figure below, 80J of heat flows into the system and the system does 30J of work.
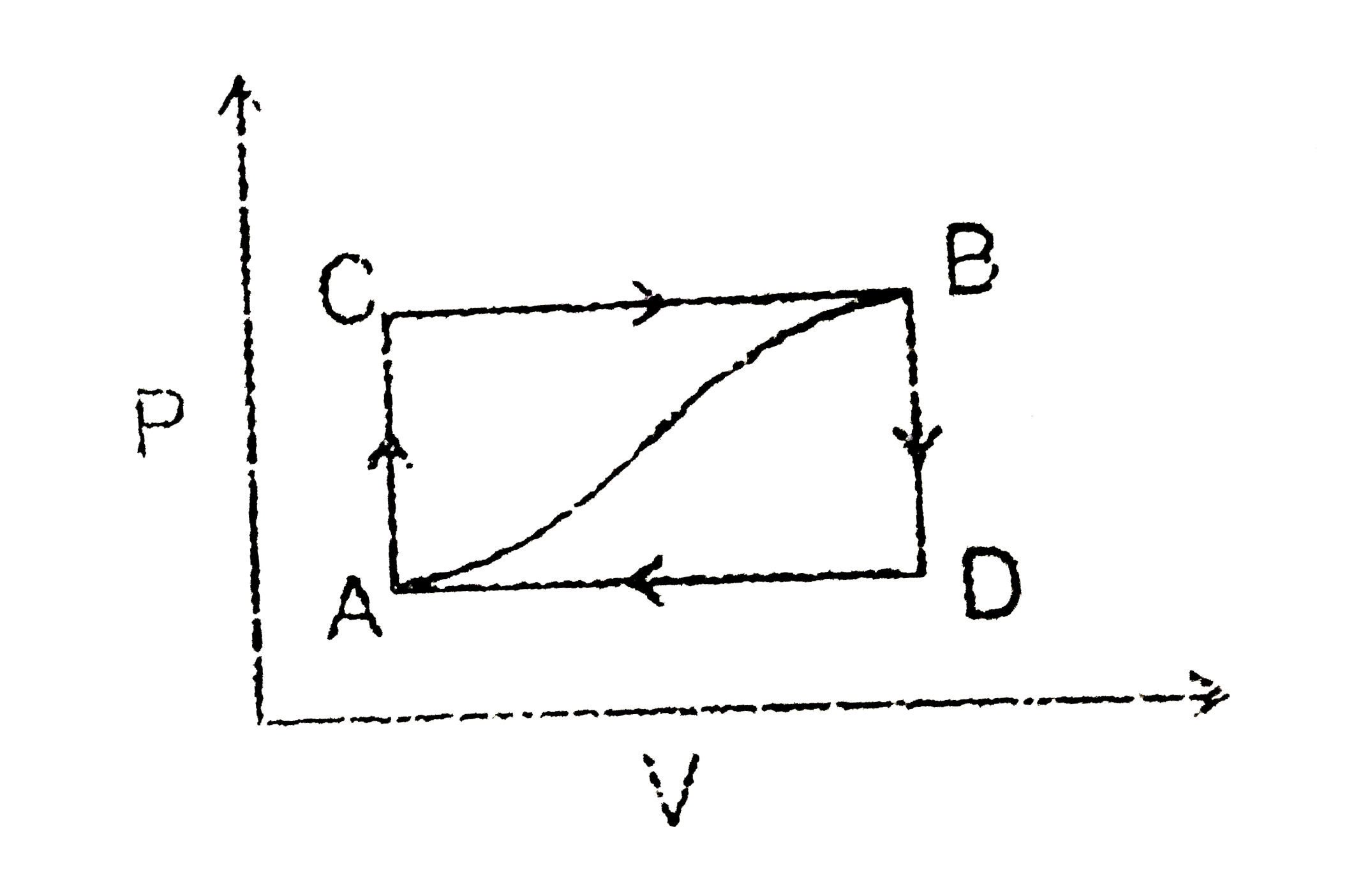
When the system is returned from state B to A along the curve path, the work done on the system is 20J.
Does the system absorb or literate heat and by how much ?
View SolutionOne mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in the figure. The change in internal energy of the gas during the transition is
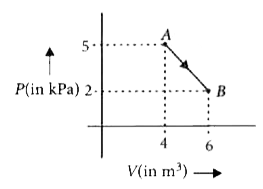
View SolutionOne mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in the figure,
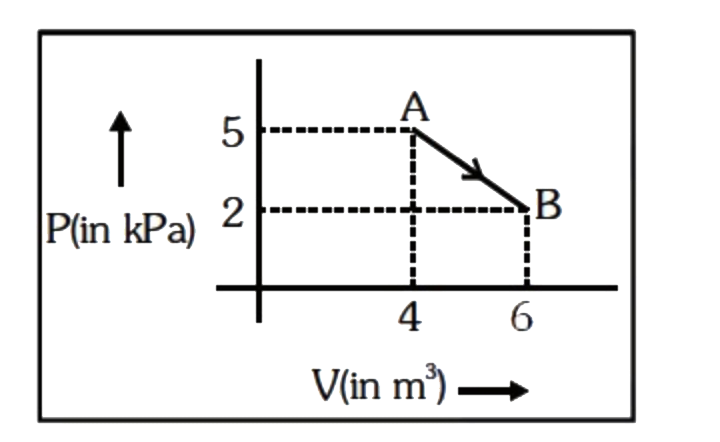
The change in internal energy of the gas during the transition is :
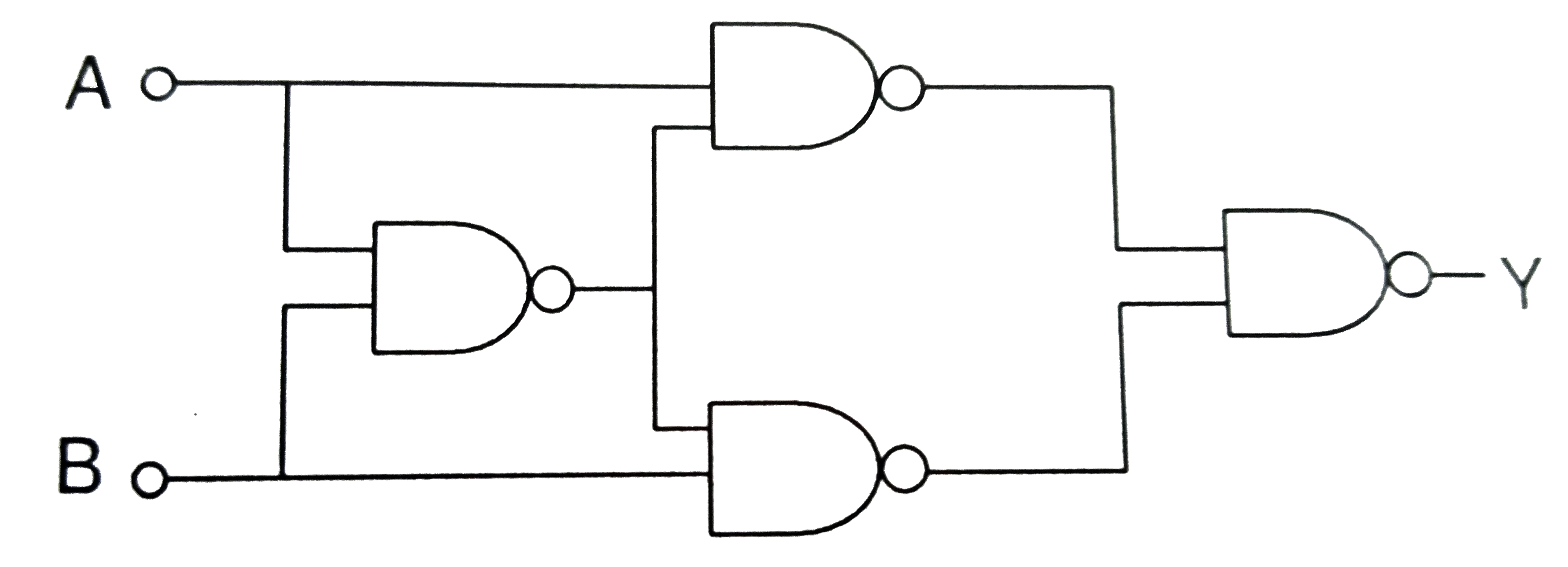
View SolutionTruth table for system of four NAND gates as shown in figure is :
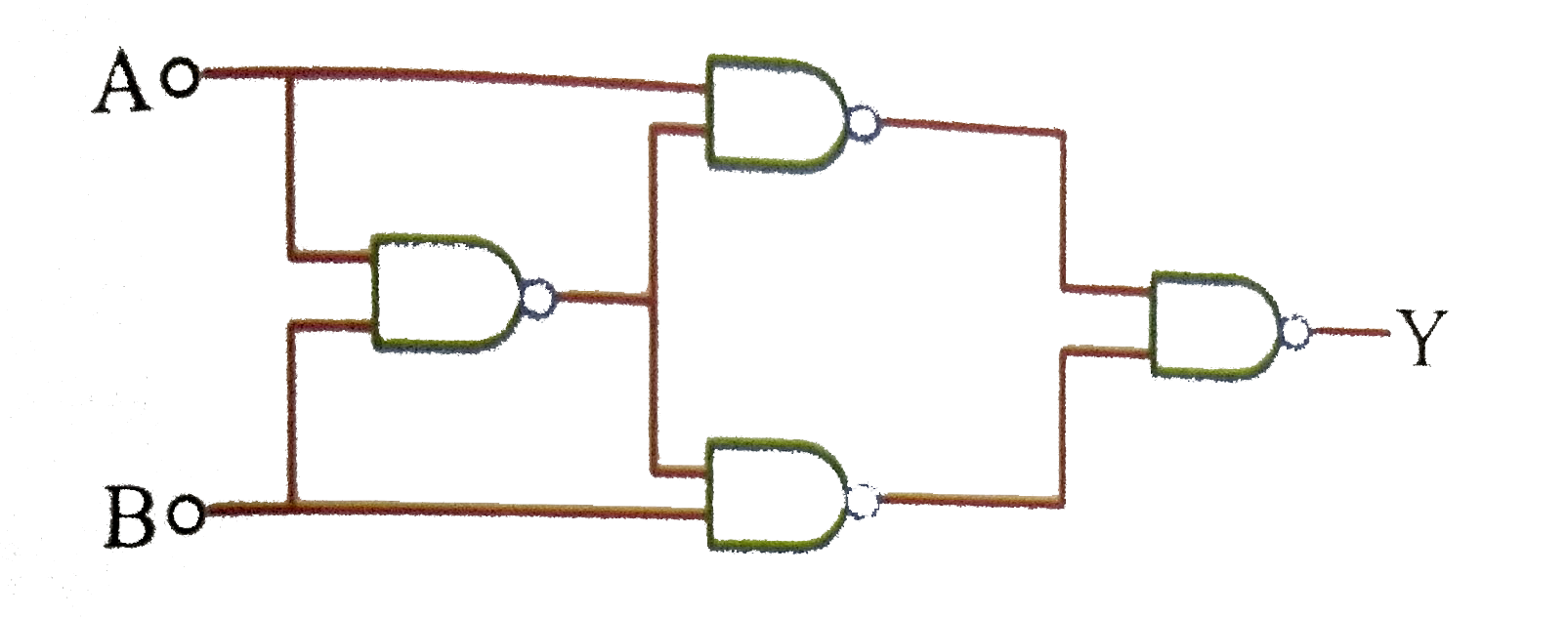 .
.
View Solution