The difference in the oxidation numbers of two types of sulphul atoms in Na2S4O6 is…..
5
4
3
2
The correct Answer is:a
Step by step video, text & image solution for The difference in the oxidation numbers of two types of sulphul atoms in Na_(2)S_(4)O_(6) is….. by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
The difference of the different oxidation number of the two of sulphur atoms in Na2S2O6 is
A+5B0C+10D−10
The difference in the oxidation states of the two types of sulphur atoms in Na2S4O6 is :
View SolutionThe difference in the oxidation numbers of the two types of sulphur atoms in Na2S4O6 is:
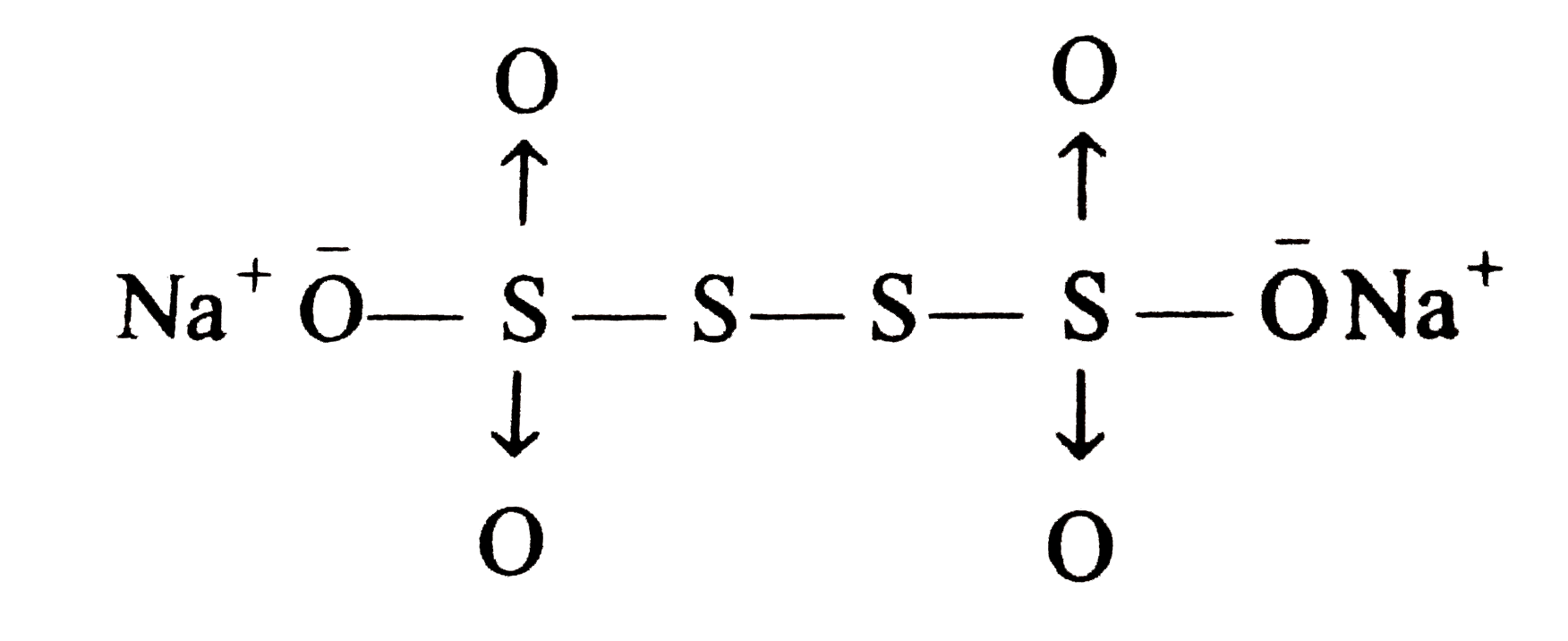
Oxidation number of sulphur atom involved in coordinate bond formation is (+5) and that of middle sulphur atom is zero. Hence the difference in oxidation number of two types of sulphur aton will be (+5).]
View SolutionThe difference in the oxidation numbers of two types of sulphul atoms in Na2S4O6 is…..
View SolutionThe difference in the oxidisation numbers of the two types of sulphur atoms in Na2S4O6 is

View SolutionThe difference in the oxidation numbers of the two types of sulphur atoms in Na2S4O6 is:

Oxidation number of sulphur atom involved in coordinate bond formation is (+5) and that of middle sulphur atom is zero. Hence the difference in oxidation number of two types of sulphur aton will be (+5).]
View SolutionThe difference in the oxidisation numbers of the two types of sulphur atoms in Na2S4O6 is

View SolutionThe difference in the oxidation states of the two types of sulphur atoms in Na2S4O6 is :
View Solution

