Indicate the nature of bonds in NaBH4.Solution in Bengali
Step by step video & image solution for Indicate the nature of bonds in NaBH_(4). by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Carbon-oxygen double bond are easily reduced by NaBH4 or LiAlH4. The actual reducing agent in these reduction is hydride ion (H−).
The metal-hydrogen bond in LiAlH4 is more polar than metal-hydrogen bond in NaBH4. As a result LiAlH4 is strong reducing agent than NaBH4. Esters, carboxylic acids, amides cannot be reducted by NaBH4.
The carbonyl group of amide to methylene group by LiAlH4.
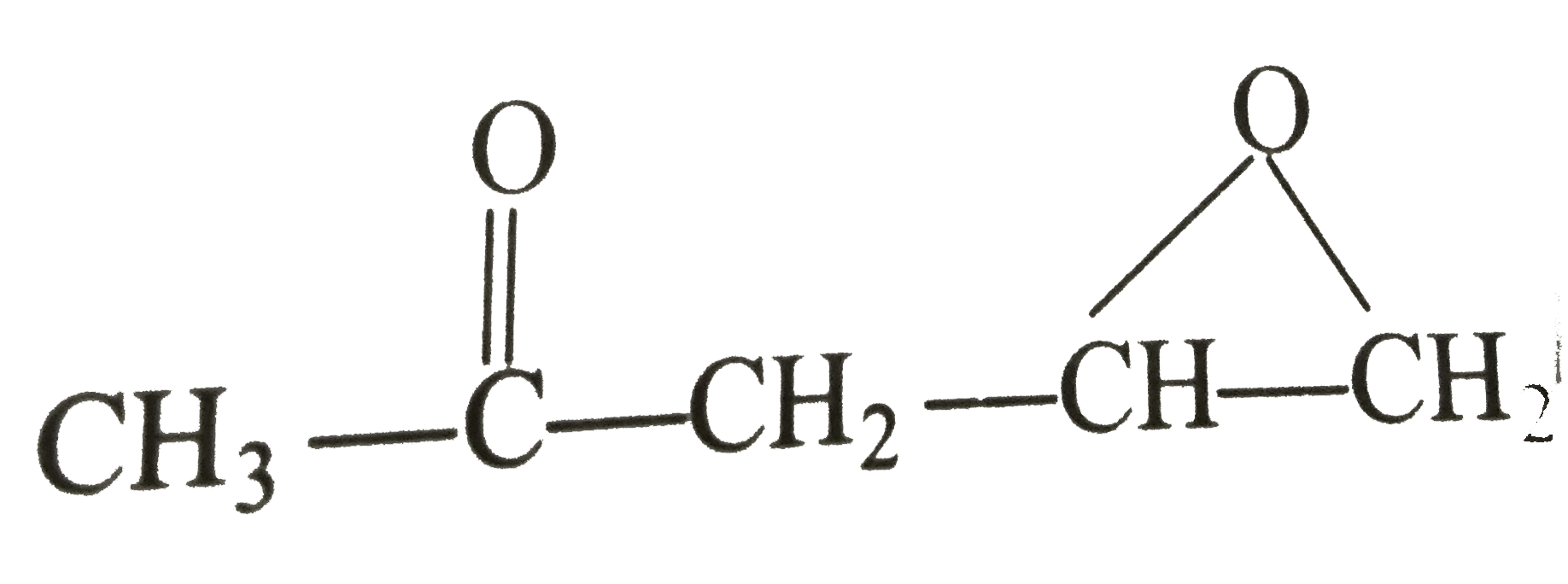 LiAlH4−−−−−−→D2O'Y', Identify 'Y':
LiAlH4−−−−−−→D2O'Y', Identify 'Y':
View SolutionCarbon-oxygen double bond are easily reduced by NaBH4 or LiAlH4. The actual reducing agent in these reduction is hydride ion (H−).
The metal-hydrogen bond in LiAlH4 is more polar than metal-hydrogen bond in NaBH4. As a result LiAlH4 is strong reducing agent than NaBH4. Esters, carboxylic acids, amides cannot be reducted by NaBH4.
The carbonyl group of amide to methylene group by LiAlH4.
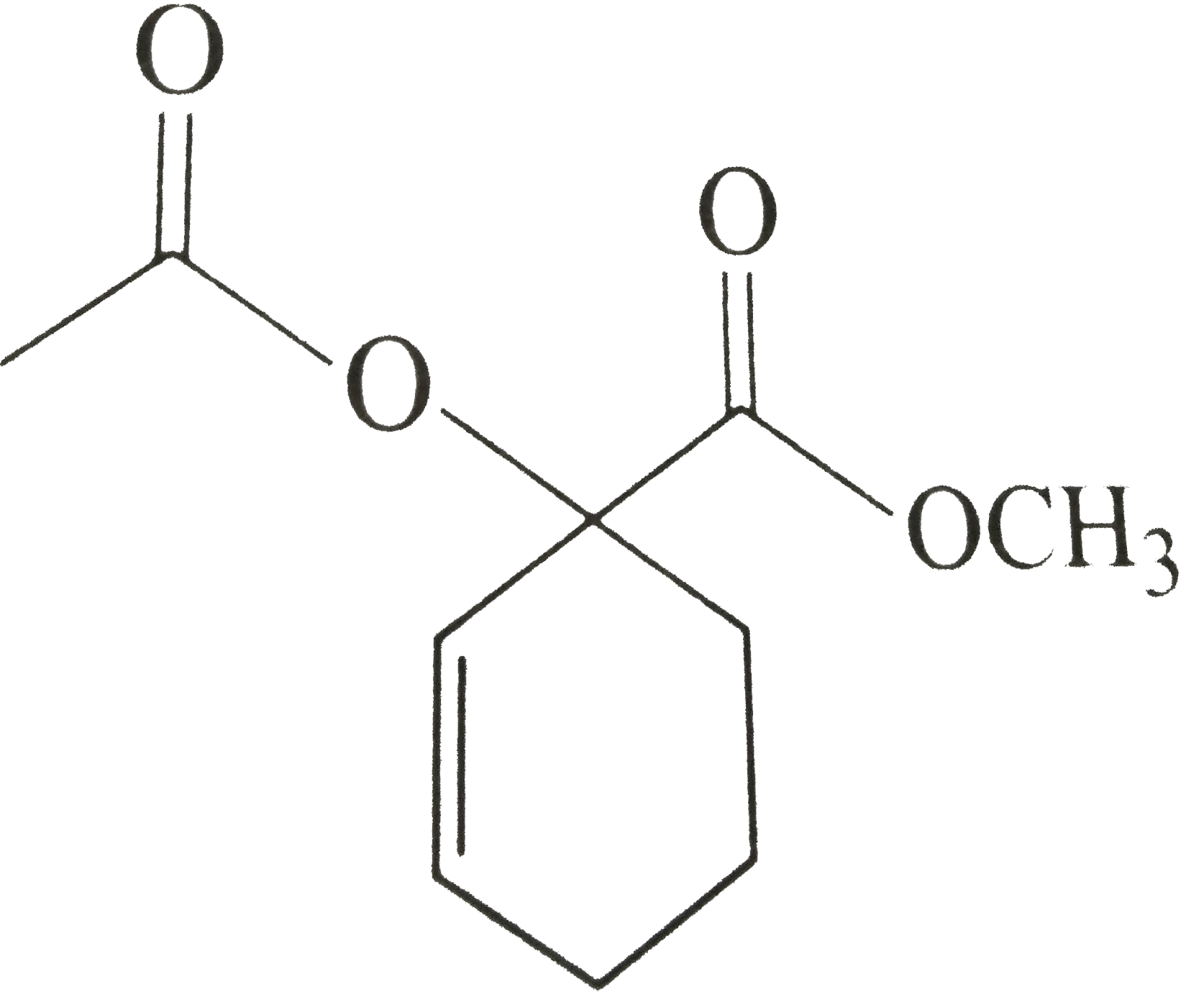 LiAlH4−−−−−−→H2O'W', Identify 'W' :
LiAlH4−−−−−−→H2O'W', Identify 'W' :
View SolutionCarbon-oxygen double bond are easily reduced by NaBH4 or LiAlH4. The actual reducing agent in these reduction is hydride ion (H−).
The metal-hydrogen bond in LiAlH4 is more polar than metal-hydrogen bond in NaBH4. As a result LiAlH4 is strong reducing agent than NaBH4. Esters, carboxylic acids, amides cannot be reducted by NaBH4.
The carbonyl group of amide to methylene group by LiAlH4.
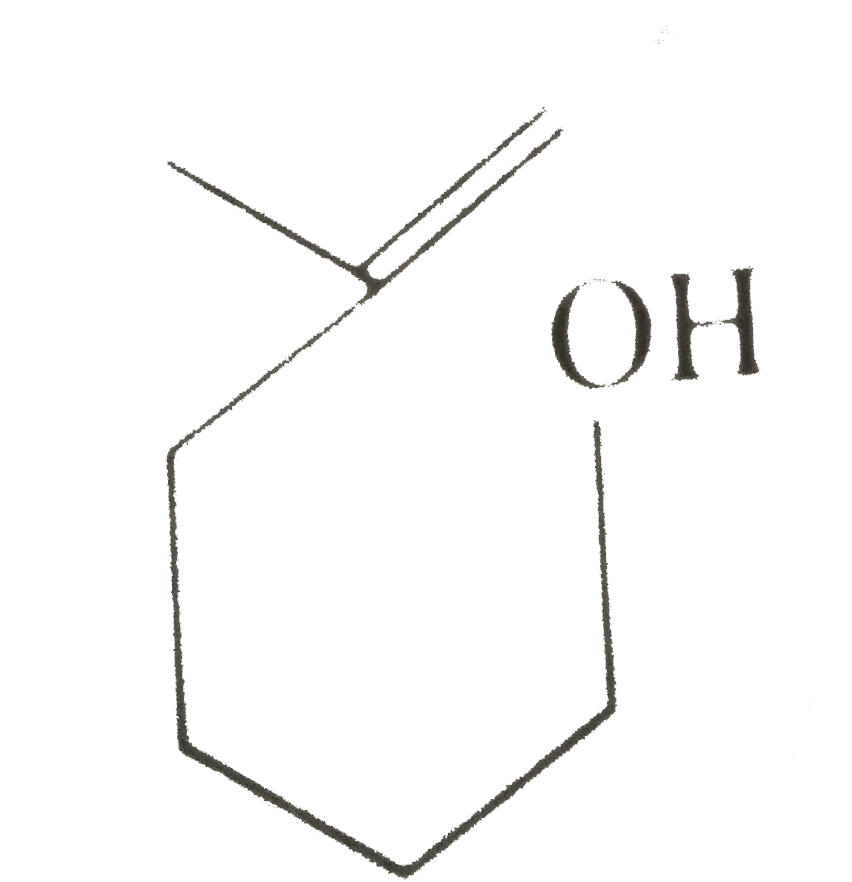 Hg(OAc)2,H2O/H+−−−−−−−−−−−−−−−−→NaBH4'Z',Identify 'Z' :
Hg(OAc)2,H2O/H+−−−−−−−−−−−−−−−−→NaBH4'Z',Identify 'Z' :
View SolutionIndicate the nature of bonding in C Cl_(4) and CaH_(2)
View SolutionIndicate the nature of bonding in diamond
View Solutionग्लूकोस NaBH4 के द्वारा अपचयित होकर देता है?
View SolutionIndicate the nature of bonds in the compound NH4Cl.
View Solution

