Carbon-oxygen double bond are easily reduced by NaBH4 or LiAlH4. The actual reducing agent in these reduction is hydride ion (H−).
The metal-hydrogen bond in LiAlH4 is more polar than metal-hydrogen bond in NaBH4. As a result LiAlH4 is strong reducing agent than NaBH4. Esters, carboxylic acids, amides cannot be reducted by NaBH4.
The carbonyl group of amide to methylene group by LiAlH4.
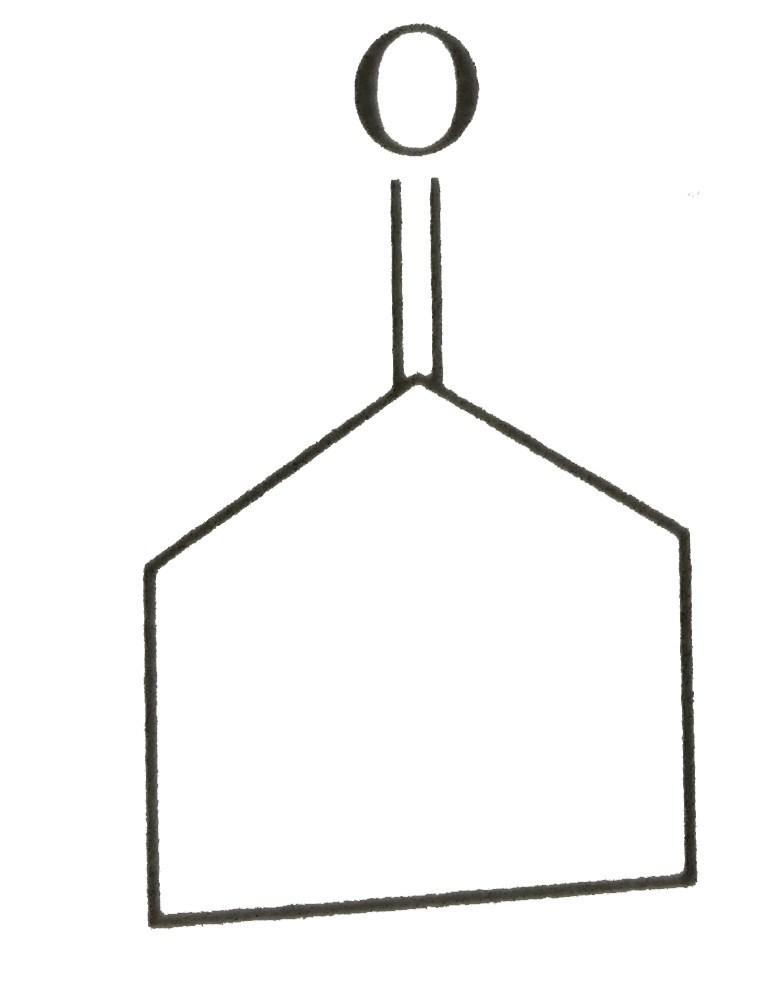 'X' , Identify X :
'X' , Identify X :
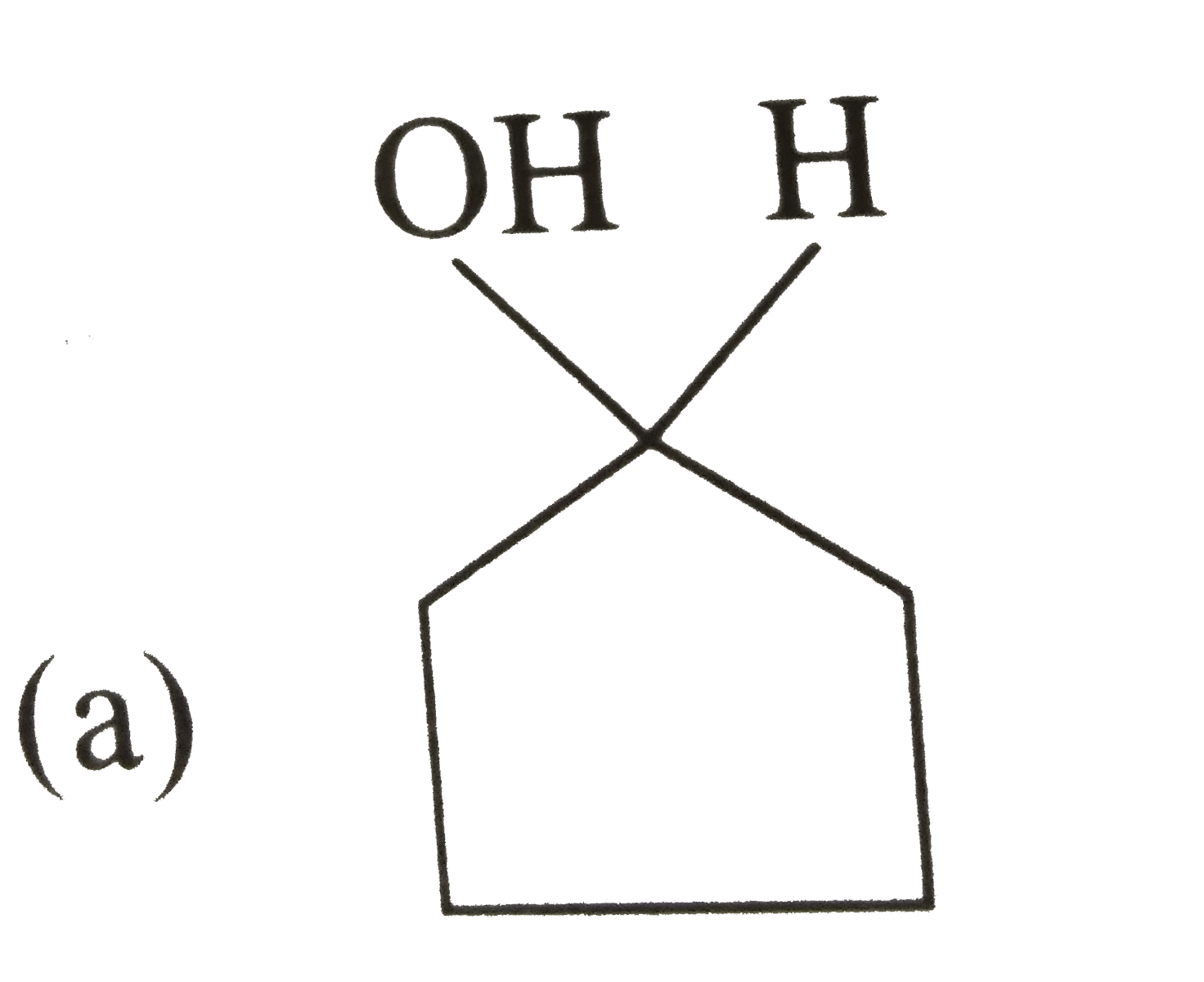
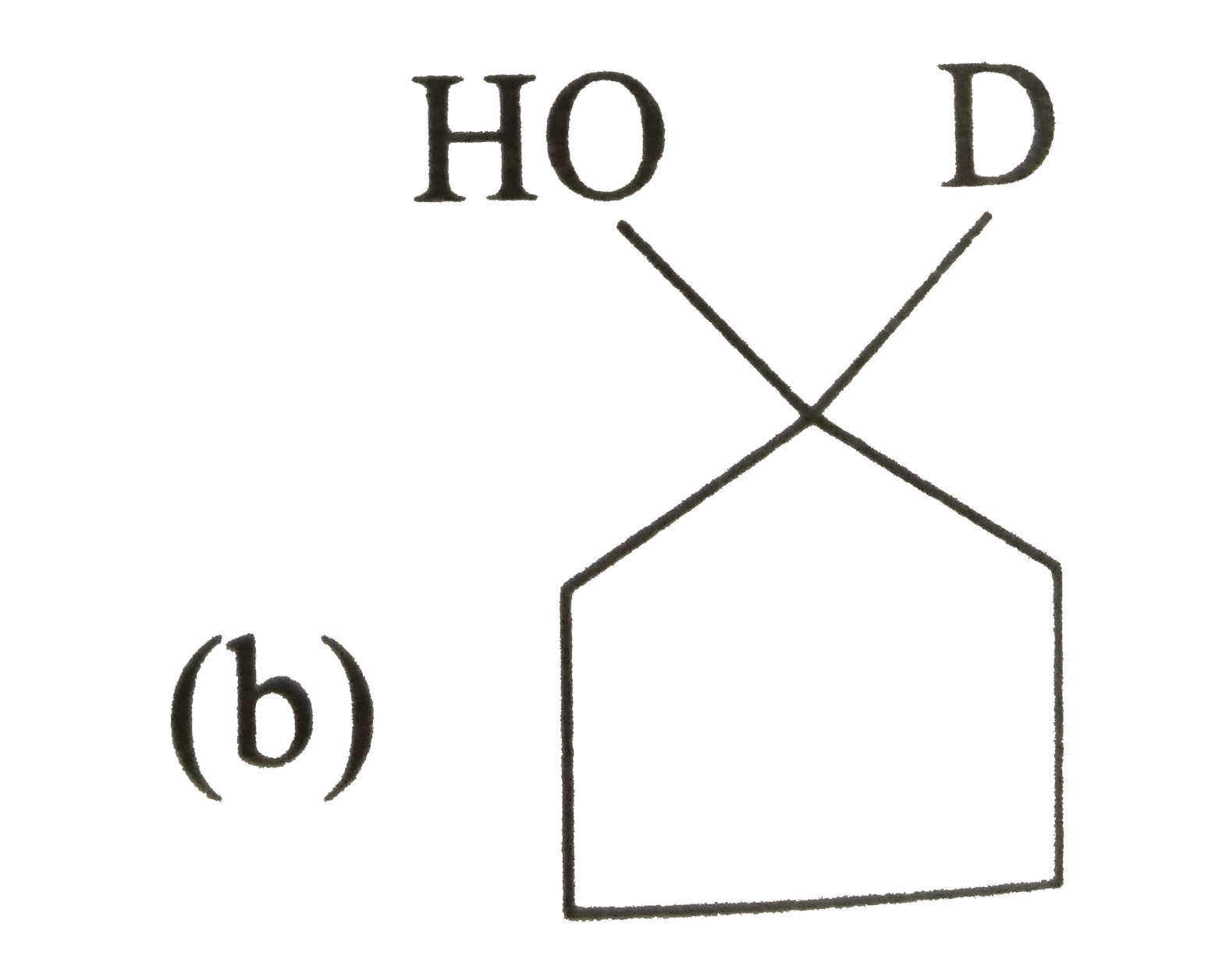
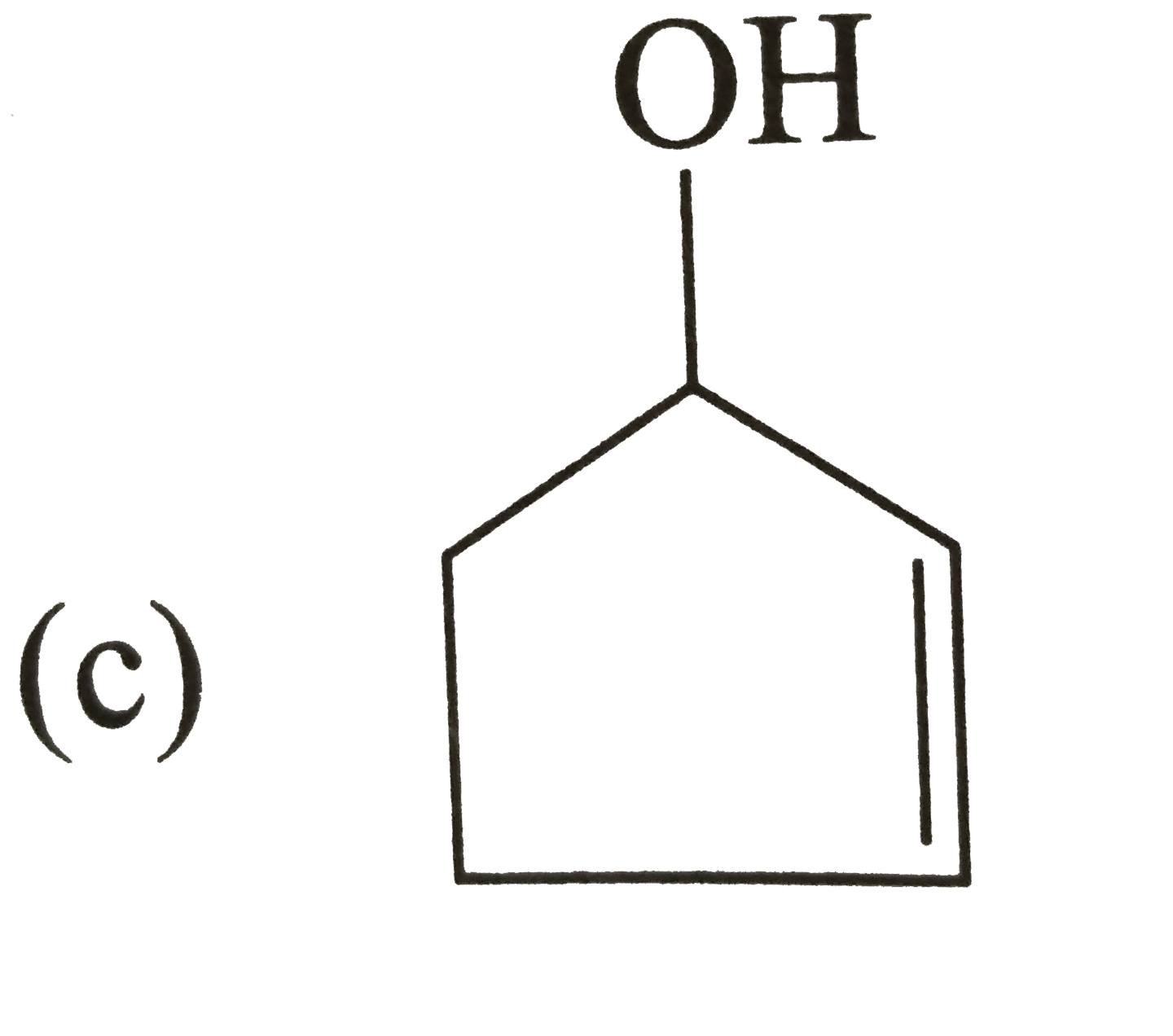
The correct Answer is:B
Topper's Solved these Questions
- ALCOHOLS, PHENOLS AND ETHERSBOOK - OP TANDONCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - Passage 5
5 Videos
- ALCOHOLS, PHENOLS AND ETHERSBOOK - OP TANDONCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - ETHERS Passage 6
5 Videos
- ALCOHOLS, PHENOLS AND ETHERSBOOK - OP TANDONCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - Passage 3
5 Videos
- ALDEHYDES AND KETONES BOOK - OP TANDONCHAPTER - ALDEHYDES AND KETONES EXERCISE - SINGLE INTEGER ANSWER TYPE QUESTIONS
10 Videos
Similar Questions
An aromatic compound (A), C9H12O was subjeted to a series of tests in the laboratory. It was found that this compound ,
(i) does not form silver mirror with Tollens' reagent.
(ii) rotates the plane of polarised light.
(iii) reacts with sodium metal to evolve hydrogen gas. ltbr> (iv) does not decolourise pink colour of bromine water.
(v) reacts with hot KMnO4 to form monocarboxylic acid 'B' which on decarboxylation gives benzene.
(vi) reacts with Lucas reagent in about 5 min.
(vii) reacts with I2 and NaOH to produce yellow coloured precipitation of the compound (C ) .
(viii) loses its optical activity due to formation of compound (D ) on reaction with Red P and HI.
The functional groups present in the compound (A) is :
View SolutionAlexander Williamson prepared diethyl by a simple method, now called as Williamson's ether synthesis . In this method, an alkyl halide is treated with sodium alkoxide prepared from sodium and alcohol.
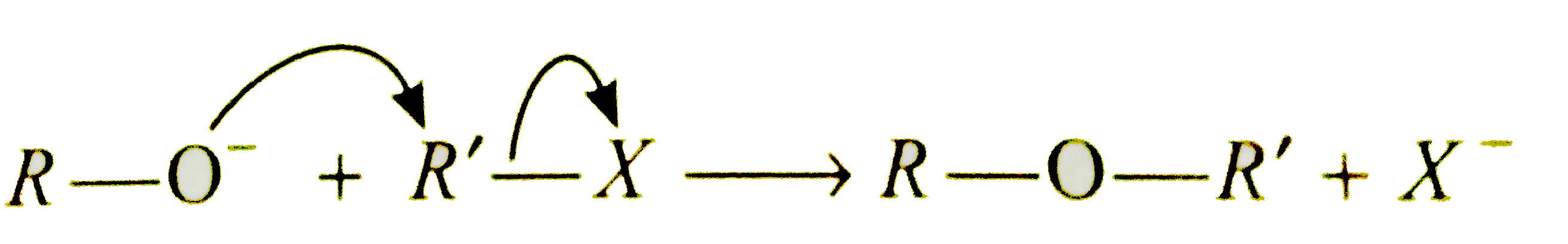
This reaction is used in the synthesis of symmetrical and unsymmetrical ethers.
It may be noted taht for preparing unsymmetrical ethers, the halide used should preferably be primary because secondary and tertiary alkyl halides may form alkenes as major product due to elimination process.
CH3−CH3∣C∣CH3−Br+Na+O−−C2H5Sodium ethoxide→CH3−CH3∣C=CH22-methylpropene+C2H5OH+NaBr
Aryl ethers or phenolic ethers can be prepared by using sodium phenoxide and alkyl halides. However, aryl halides and sodium alkoxide cannot be used for preparing phenolic ethers because aryl halides are less reactive towards nucleophilic substitution reaction
Arrange the following halides in decreasing order of reactivity towards Williamson's ether synthesis :
CH3CH2CH2Cl(u), CH3CH2CH2Br(v),
(CH3)3C−CH2Br(w), H2C=CH−CH2Cl(x)
View SolutionAlcohols are acidic in nature because hydrogen is attached to oxygen which is an electronegative element. Alcohol is weaker acid than water because OH− ion is more stable than R−O− ion. R−O− is less stable, due to +I effect of the alkyl group. Acidity of alcohols increases with increase in the stability of acid anion.
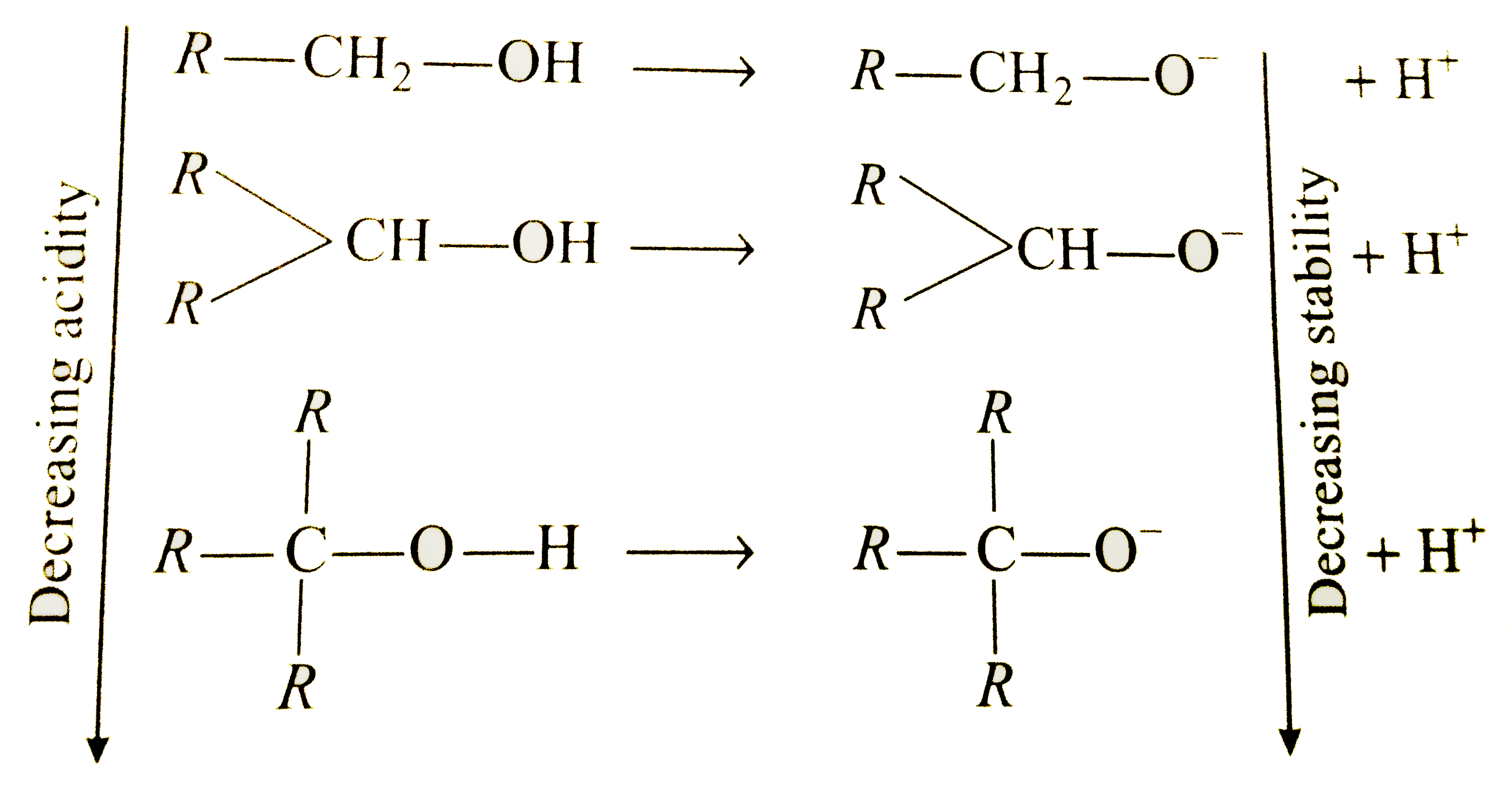
Some alcohol like CH3−OH,CF3−CH2−OH,(CF3)3C−OH ae more acidic than water.
Which among the following is the most acidic ?
View SolutionIsomeric aldehydes and ketones having the formula C5H10O are :
View SolutionWhich of the following is reduced most easily to an alkane by NaBH4orLiAlH_(4)?
View SolutionThe maximum number of moles of carbonyl compound (aldehyde or ketone) that can be reduced by one mole of NaBH4 or LiAlH4 is
View SolutionMatch the column
Column IColumn II(A) acid chloride(p) Readily halogenated at αposition (B) Acid anhydride(q) Can be reduced into alcohol by LiAlH4in ether(C) Ester(r) Can be reduced into alcohol by NaBH4in ethanol(D) Acid amide(s) Can undergo catalytic hydrogenation
View SolutionAssertion : LiAlH4 is more reactive and less selctive than NaBH4 as a reducing agent.
Reason : LiAlH4 reduces both aldehydic group and double bond in conjugate position while NaBH4 does not.
View SolutionChoose the amide which on reduction with LiAlH_(4) yields a secondary amine
View SolutionIdentify the product?
(i). NaBH4←−−−−Me−CHOLiAIH4−−−−→
(ii). NaBH4←−−−−Me2C∞verset(LiAlH4)→
(iii). NaBH4−−−−→Me−COClLiAlH4−−−→
(iv). NaBH4←−−−−Me−COOE→verset(LiAlH4)→
(v). NaBH4−−−−→Me−COOHLiAlH4−−−→
(vi). NaBH4←−−−−Me−COOMeLiAlH4−−−→
(vii). NaBH4←−−−−Me−CONH2LiAlH4−−−→
(viii). NaBH4←−−−−Me−CONH−MeLiAlH4−−−→
(ix). NaBH4←−−−−Me−CONMe2LiAlH4−−−→
(x). NaBH4←−−−−Me−CH=NHLiAlH4−−−→
(xi). NaBH4←−−−−CH3−CH=CH2LiAlH4−−−→
View SolutionPropiophenone is reduced by NaBH4 gives
View SolutionGlucose on reduction with NaBH_(4) gives
View SolutionGlucose on reduction with NaBH4 gives
View SolutionIndicate the nature of bonds in NaBH4.
View SolutionAssertion(A): Reduction of crotonaldehyde is preferbaly carried in the presence of LiAlH4.
Reason(R): LiAlH4 does not reduce carbon-carbon double bond in the carbonyl compound.
View SolutionAcetic acid can be reduced by LiAlH_(4) to :
View Solution

