For a definite mass of an ideal gas at constant pressure, V versus 1P plot is a-Solution in Bengali
parabola
straight line
hyperbola
rectangular hyperbola
Step by step video & image solution for For a definite mass of an ideal gas at constant pressure, V versus 1/P plot is a- by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
For 1 mol ofan ideal gas at a constant temperature T, the plot of (log P) against (log V) is a (P: Pressure, V: Volume)
View SolutionA plot of volume (V) versus temperature (T) for a gas at constant pressure is a straight line passing through the origin. The plots at different values of pressure are shown in figure. Which of the following order of pressure is correct for this gas ?
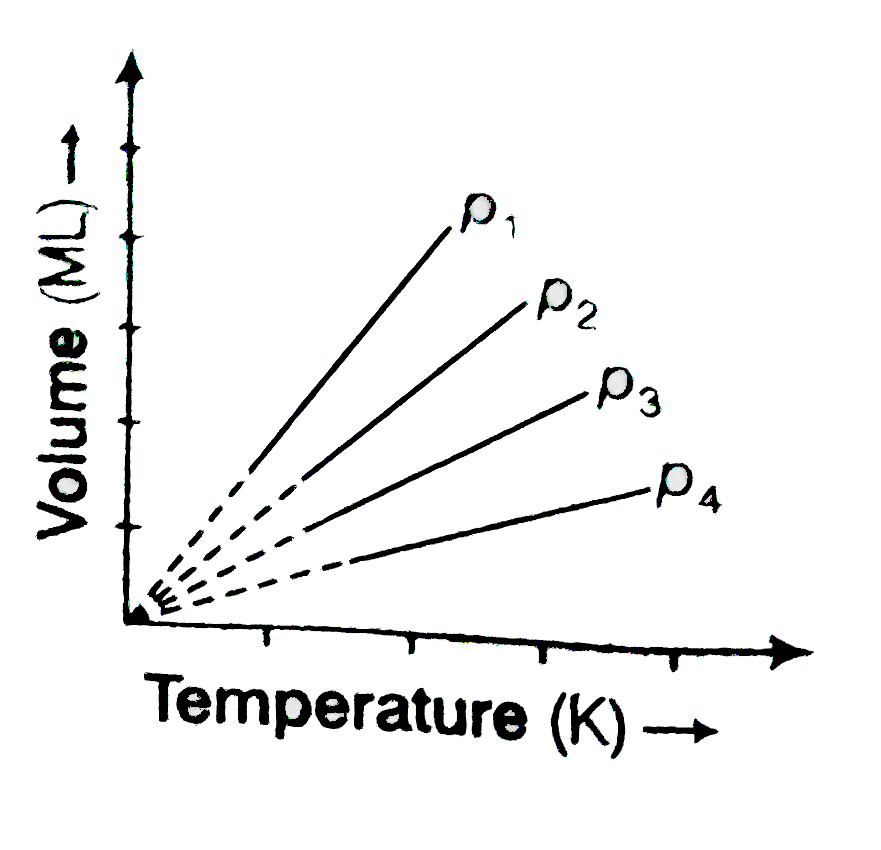
View SolutionSTATEMENT-1 : Plot of P vs 1/V (volume) is a straight line for an ideal gas. STATEMENT-2 : Pressure is directly proportional to volume for an ideal gas.
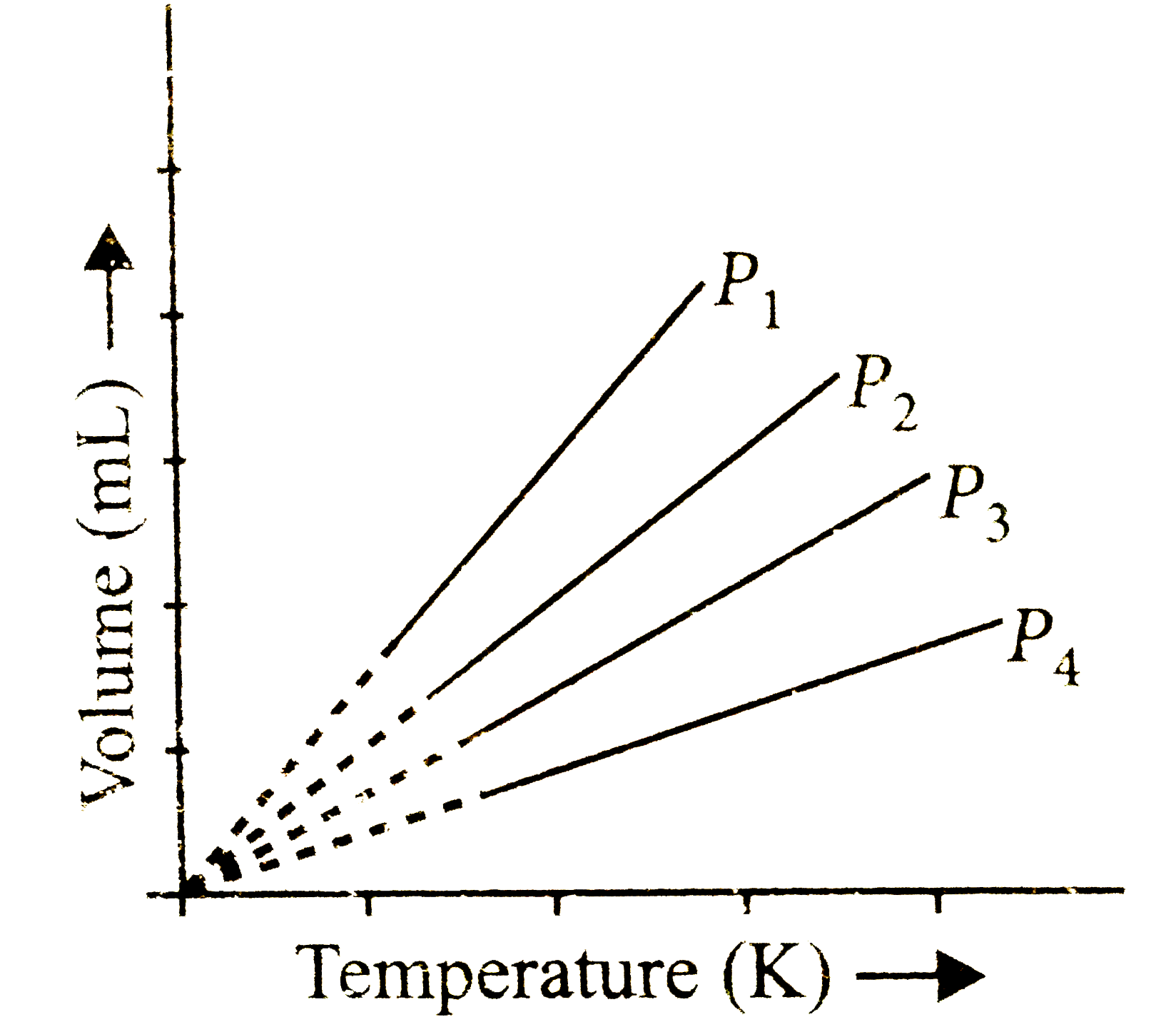
View SolutionA plot of volume (V) versus temperature (T) for a gas at constant pressure is a straight line passing through the origin . The plots at different values of pressure are shown in figure .
Which of the following order of pressure is correct for this gas ?
View SolutionP-V diagram of an ideal gas for a process ABC is as shown in the figure.
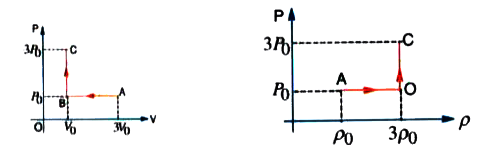
Plot pressure versus density graph of the gas for the process ABC.
View SolutionPressure versus temperature graph of an ideal gas at constant volume V is shown by the straight line A. Now mass of the gas is doubled and the volume is halved, then the corresponding pressure versus temperature graph will be shown by the line
View SolutionFor a definite mass of an ideal gas at constant temperature, V versus 1/Pplot is a
View Solution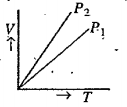
V versus T curves at constant pressure P1 and P2 for an ideal gas are shown in fig.
View Solution
