Which of the following complex does not show geometrical isomerism ?
A
[Co(NH3)4Cl2]+
B
[Co(NH3)3(NO2)3]
C
[Cr(en)3]3+
D
[Pt(gly)2]
Video Solution
Text Solution
The correct Answer is:C
[M(AA)3] does not show G.I.
|
Topper's Solved these Questions
- COORDINATION COMPOUNDSBook:GRB PUBLICATIONChapter:COORDINATION COMPOUNDSExercise:G. Organometallic Compounds, Synergic Bonding
Explore 52 Videos
- COORDINATION COMPOUNDSBook:GRB PUBLICATIONChapter:COORDINATION COMPOUNDSExercise:REASONING TYPE
Explore 59 Videos
- COORDINATION COMPOUNDSBook:GRB PUBLICATIONChapter:COORDINATION COMPOUNDSExercise:E. Stability, Colour and Magnetic Properties of Complexes
Explore 60 Videos
- CHEMICAL KINETICSBook:GRB PUBLICATIONChapter:CHEMICAL KINETICSExercise:Subjective Type
Explore 63 Videos
- D-BLOCK ELEMENTSBook:GRB PUBLICATIONChapter:D-BLOCK ELEMENTSExercise:Subjective Type
Explore 18 Videos
Similar Questions
Knowledge Check
Which of the following compound does not show geometrical isomerism?
A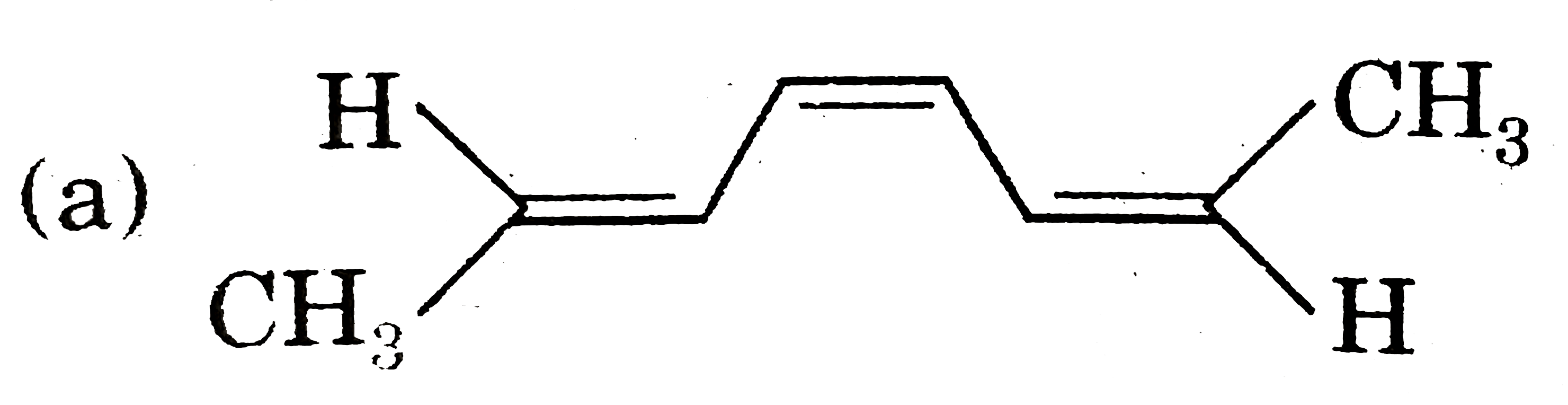 B
B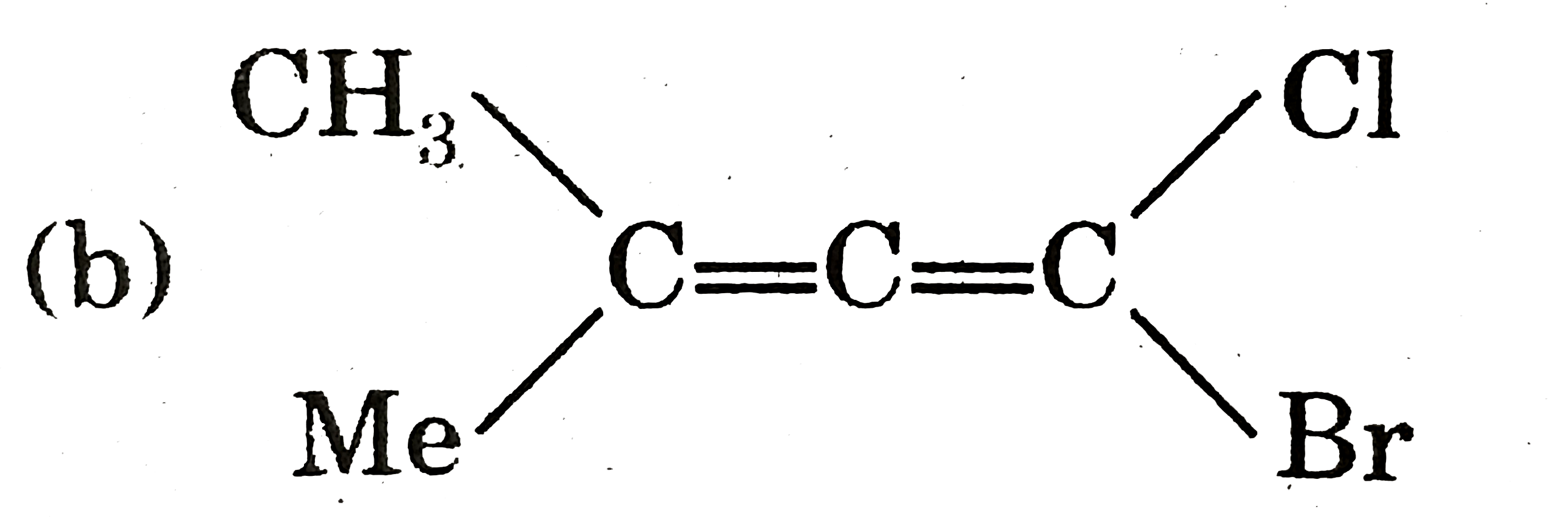 C
C D
D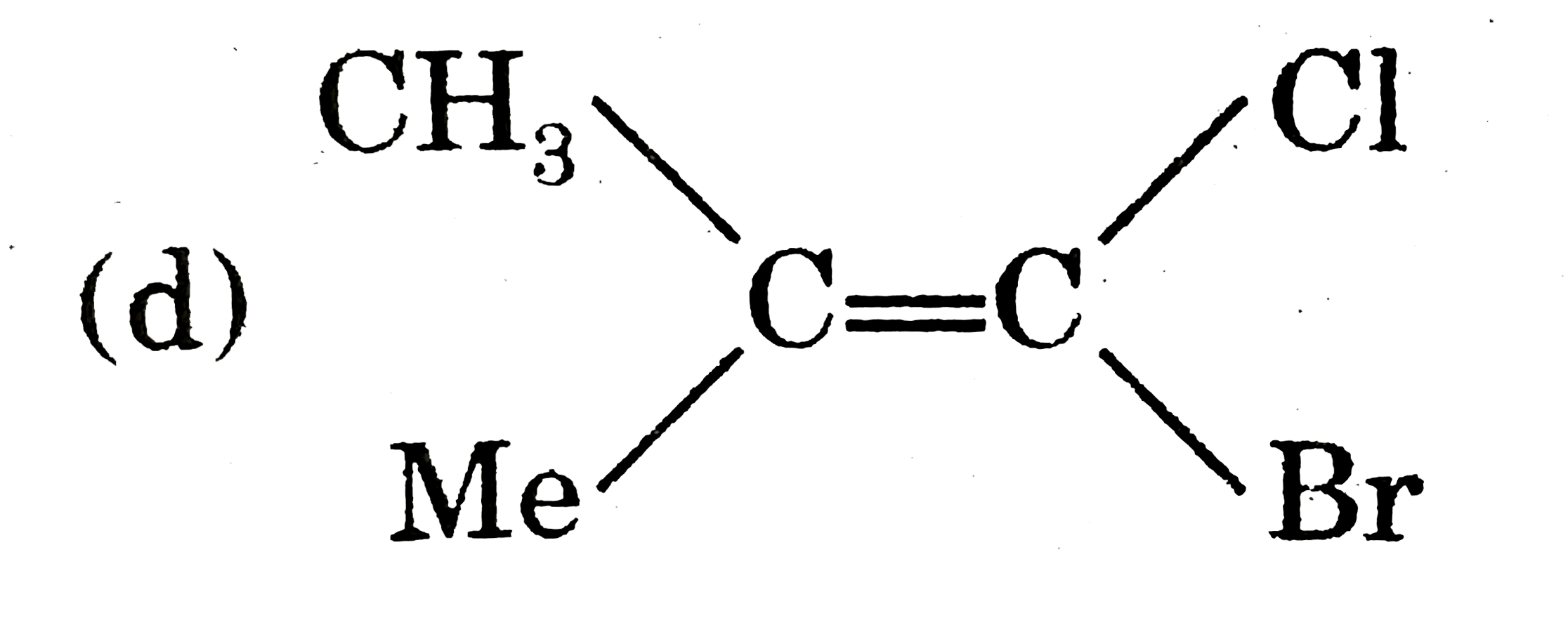
Which of the following complex does not show optical isomerism
A[Cr(C2O4)3]3−BCis [Pt(Br)2(en)2]2+C[CrCl2(NH3)2en]+D[Cr(NH3)4(SO4)2]+Which of the following complexes will show geometrical isomerism ?
APotassium tris(oxalato) chromate (III)BPentaaquachlorochromium (III) chlorideCAquachlorobis (ethylenediamine) cobalt (II) chlorideDPotassium aminetrichloroplatinate (II)
Which of the following does not show geometrical isomerism?
View SolutionWhich of the following does not show geometrical isomerism?
View SolutionWhich of the following does not show geometrical isomerism?
View SolutionWhich of the following does not show geometrical isomerism?
View SolutionWhich of the following does not show geometrical isomerism
View Solution
