The complexes having different CO bond length is/are :
Mn2(CO)10
Co2(CO)8
Fe2(CO)9
Fe(CO)5
The correct Answer is:B, C, D
(a) Mn2(CO)10 has no bridge bond, hence all C-O bond length are equal.
(b) 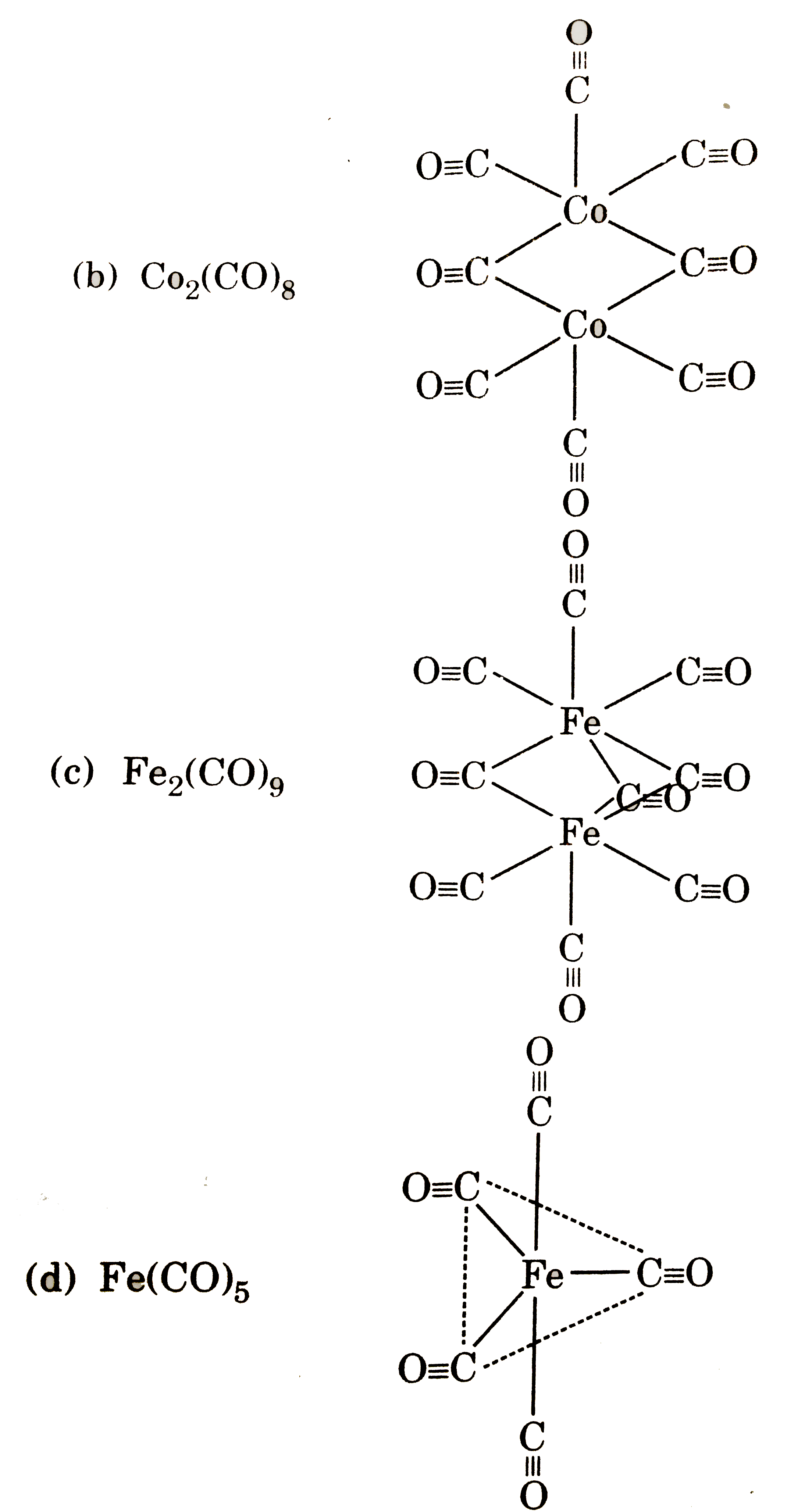
Topper's Solved these Questions
- COORDINATION COMPOUNDSBOOK - GRB PUBLICATIONCHAPTER - COORDINATION COMPOUNDSEXERCISE - COMPREHENSION 1
3 Videos
- COORDINATION COMPOUNDSBOOK - GRB PUBLICATIONCHAPTER - COORDINATION COMPOUNDSEXERCISE - COMPREHENSION 2
3 Videos
- COORDINATION COMPOUNDSBOOK - GRB PUBLICATIONCHAPTER - COORDINATION COMPOUNDSEXERCISE - REASONING TYPE
59 Videos
- CHEMICAL KINETICSBOOK - GRB PUBLICATIONCHAPTER - CHEMICAL KINETICSEXERCISE - Subjective Type
63 Videos
- D-BLOCK ELEMENTSBOOK - GRB PUBLICATIONCHAPTER - D-BLOCK ELEMENTSEXERCISE - Subjective Type
18 Videos
Similar Questions
Match the entries listed in Column I with appropriate entries listed in Column II
AK3[Fe(CN)5(CO)]pComplex having lowest bond length of CO ligandBK[PtCl3(C2H4)]qFollow rule of EANCNa[Co(CO)4]rComplex involvedin synergic bondingDV(CO)6sComplex having highest bond length of Co ligand
View SolutionWhich of the following complex is having highest M−C bond order?
(1) [Ni(CO)4]
(2) [Fe(CO)5]
(3) [V(CO)6]−
(4) [Mn(CO)6]+
View Solution
Knowledge Check
Select the compound having all equivalent bond lengths :
APCl5BBF3CPF5DIF7Which of the following complexes has/have more C-O bond length than [Ni(CO)4]?
ANa[Co(CO)4]B[Fe(CO)4]2−CBoth 1 and 2DNone of theseWhich of the following complexes has/have more C – O bond length than [Ni(CO)4] ?
ANa[Co(CO)4]B[Fe(CO)4]2−CBoth (1) and (2)DNone of these
Which of the following molecules or ions has different bond lengths ?
View SolutionThe species having bond order different from that in CO is
View SolutionThe species having bond order different from that in CO is
View SolutionWhich of following molecule is having shortest bond length
View SolutionDifferent types of bond are formed in the chemical compound. These bond have different strength and bond energies associated with them. These bond are formed with atoms in different enviroments.
Which of the following bond has highest bond energy ?
View Solution

