Find the correct order of dipole moment-
A
HF<HCl
B
CH3F>CH3Cl
C
CH2Cl2>CHCl3
D
NF3>NH3
Video Solution
Text Solution
(I)H−FμαE.N.
H−Clμ⇒H−F>HCl
(2) CH3−F<CH3−Cl
Distance factor dominats over charge factor
μ=q×d
(3) CH2Cl2>CHCl3
(4) NF3>NH3
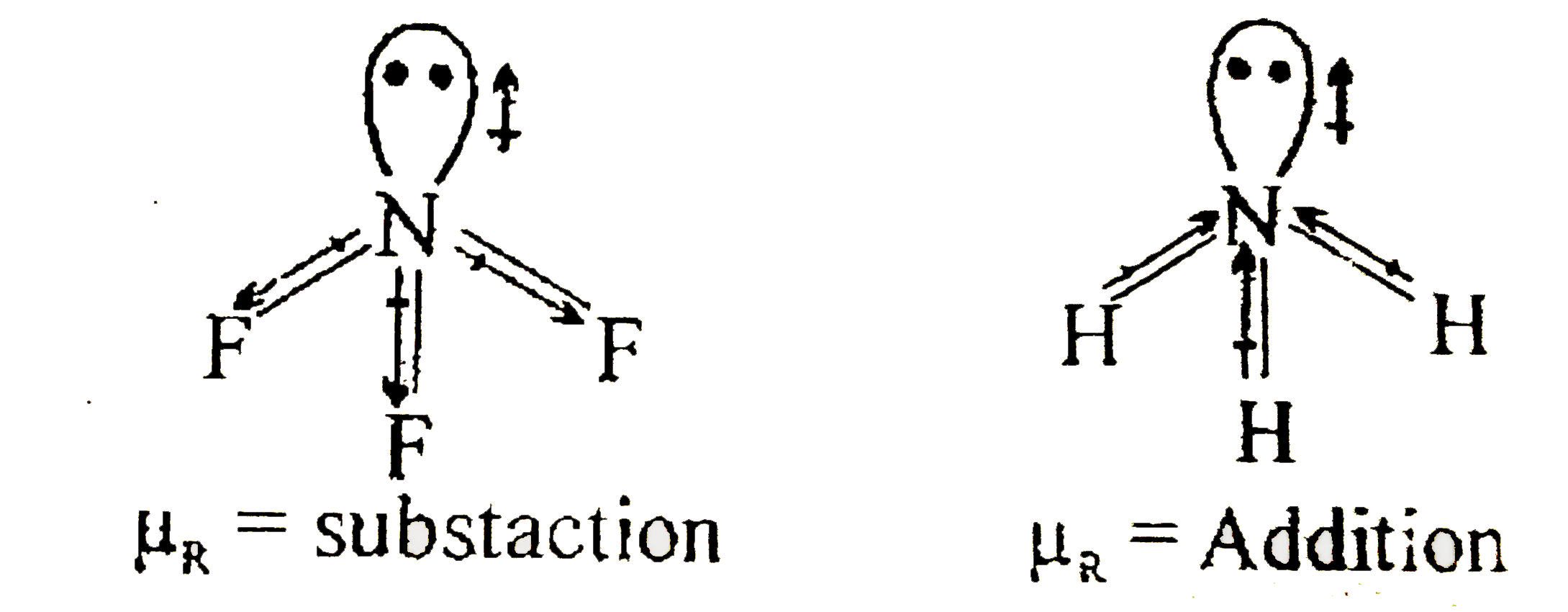
|
Similar Questions
Knowledge Check
The correct order of dipole moment is
ACH4<NF3<NH3<H2OBNF3<CH4<NH3<H2OCNH3<NF3<CH4<H2ODH2O<NH3<NF3<CH4The correct order of dipole moment is :
ACH4<NF3<NH3<H2OBNF3<CH4<NH3<H2OCNH3<NF3<CH4<H2ODH2O<NH3<NF3<CH4The correct order of dipole moment is :
ACH4<NF3<NH3<H2OBNF3<CH4<NH3<H2OCNH3<NF3<CH4<H2ODH2O<NH3<NF3<CH4
Identify the correct order of dipole moment in the following:
View SolutionWhich of the following is the correct order of dipole moment?
View SolutionThe correct order of dipole moment is :
View SolutionThe correct order of dipole moment is :
View SolutionThe correct order of dipole moment is :
View Solution
