Which of the following molecules has a dipole moment of zero?
A
HCN
B
CH2Cl2
C
SO2
D
CO2
Video Solution
Text Solution
The correct Answer is:D
|
Answer
Step by step video, text & image solution for Which of the following molecules has a dipole moment of zero? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMICAL BONDING-IBook:GRB PUBLICATIONChapter:CHEMICAL BONDING-IExercise:F.Resoncance
Explore 29 Videos
- CHEMICAL BONDING-IBook:GRB PUBLICATIONChapter:CHEMICAL BONDING-IExercise:G.Hydrogen Bonding
Explore 50 Videos
- CHEMICAL BONDING-IBook:GRB PUBLICATIONChapter:CHEMICAL BONDING-IExercise:D.
Explore 54 Videos
- CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSBook:GRB PUBLICATIONChapter:CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDSExercise:Subjective type
Explore 19 Videos
- CHEMICAL KINETICSBook:GRB PUBLICATIONChapter:CHEMICAL KINETICSExercise:Subjective Type
Explore 63 Videos
Similar Questions
Knowledge Check
Which of the following molecules has no dipole moment ?
ACH3ClBCHCl3CCH2Cl2DCCl4Which of the following molecules have dipole moment?
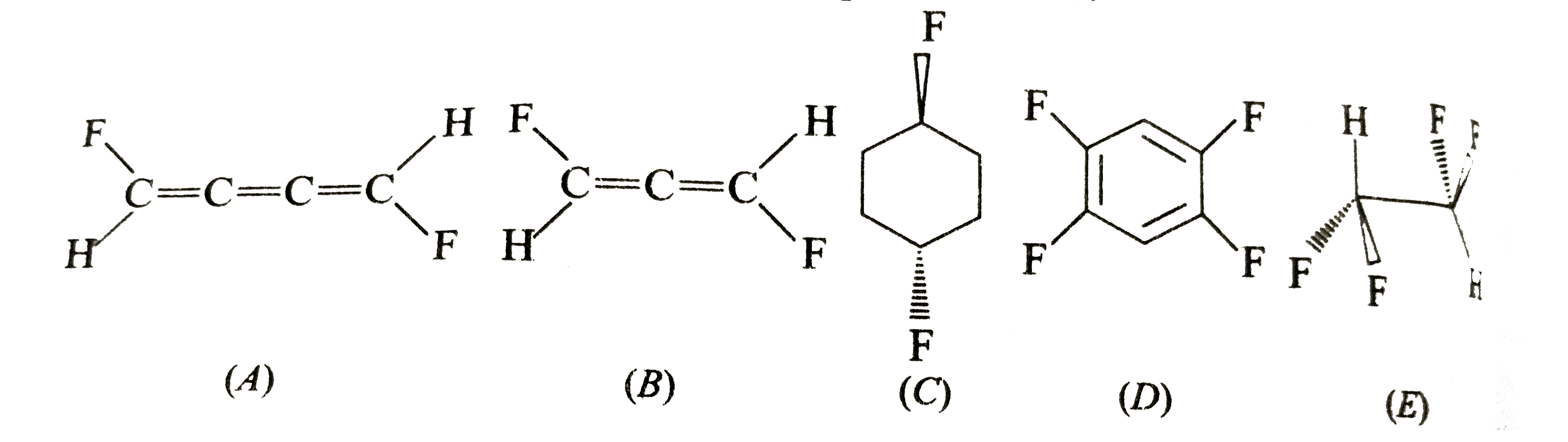 AA and DBB and CCC and EDB and E
AA and DBB and CCC and EDB and EWhich of the following molecule has net dipole moment?
ACO2BH2OCBF3DCH4
Which of the following molecule has net dipole moment?
View SolutionWhich of the following molecules has zero dipole moment ?
View SolutionWhich of the following molecule has a net dipole moment?
View SolutionWhich of the following molecule has some dipole moment :-
View SolutionWhich of the following molecule have zero dipole moment ?
View Solution
