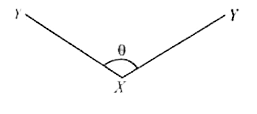
Which bond angle, θ would result in the maximum dipole moment for the triatomic molecule XY2 shown below? ltvegt 
A
θ=90∘
B
θ=120∘
C
θ=150∘
D
θ=180∘
Text Solution
The correct Answer is:A
μnet=√μ21+μ22+2μ1μ2cosθ
|
Similar Questions
Knowledge Check
Which bond angle θ would result in the maximum dipole moment for the triatomic molecule XY2 as shown below?
 A90∘B120∘C150∘D180∘
A90∘B120∘C150∘D180∘Which bond angle θ would result in the maximum dipole moment for the triatomic YXY?
Aθ=90∘Bθ=120∘Cθ=150∘Dθ=180∘Which bond angle, θ would result in the maximum dipole moment for the triatomic molecule XY2 ?
Aθ=90∘Bθ=120∘Cθ=150∘Dθ=180∘
Which bond angle 0 would result in the maximum dipole moment for the triatomic molecule XY2 as shown in figure?
View SolutionWhich of the following molecules has the maximum dipole moment ?
View SolutionWhich of the following molecules has the maximum dipole moment?
View SolutionWhich of the following molecules has the maximum dipole moment?
View SolutionWhich of the following planar molecule has maximum dipole moment?
View Solution

