What is enthalpy of a reaction? Explain the standard enthalpy of a reaction.Solution in Telugu
Video Solution
Text Solution
The enthalpy of a reaction is defined as the enthalpy change accompanying the chemical reaction when the molar quantities of reactants and products are the same as indicated in the chemical equation. It is also known as heat of reaction. It is represented by ΔH.
The enthalpy change at the standard state conditions is called standard enthalpy of the reaction. It is denoted by ΔrHθ. The superscript (θ) represented standard state.
Standard state of a substance is its most stable state at one bar presume and 208K.
|
Topper's Solved these Questions
Similar Questions
What are the postulates of Bohr's model of hydrogen atom?
View SolutionWhat is Charya Enthalpi? Explain the standard action enthalpy.
View SolutionExplain the standard enthalpy of reactions.
View SolutionDefine and illustrate standard enthalpy of reaction.
View SolutionWhat is standard enthalpy of reaction give example
View SolutionExplain the step for calculation of standard enthalpy of reaction from standard enthalpy of formation.
View SolutionCalculate standard enthalpy of reaction
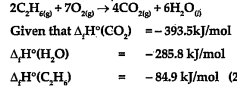
View Solutionमानक संभवन एंथैल्पी को परिभाषित कीजिए । यह अभिक्रिया की मानक एंथैल्पी से किस प्रकार भिन्न है ?
View Solution
