Explain standard enthalpy of reaction.Solution in Kannada
Video Solution
Text Solution
Standard enthalpy of reaction is the change in enthalpy that occurs, when the number of moles of the reactants indicated by the balanced chamical equation have completely reached under standard conditions, that is, 298 K and one bar pressure [ΔH∘]
Example : H2(g)+12O2(g)→H2O(l),ΔH∘=−285.8kJ.
|
Similar Questions
Explain the standard enthalpy of reactions.
View SolutionExplain standard enthalpy of formation
View SolutionExplain the step for calculation of standard enthalpy of reaction from standard enthalpy of formation.
View SolutionCalculate standard enthalpy of reaction
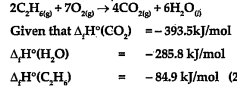
View Solutionमानक संभवन एंथैल्पी को परिभाषित कीजिए । यह अभिक्रिया की मानक एंथैल्पी से किस प्रकार भिन्न है ?
View SolutionChoose the reaction, for which the standard enthalpy of reaction is equal to the standard enthalpy of formation:
View SolutionWhat is enthalpy of a reaction? Explain the standard enthalpy of a reaction.
View SolutionDefine and explain the standard enthalpy of vapourisation of sublimation.
View Solution
