In the above graph speed is high at
A
B
C
speed is equal
The correct Answer is:B
Similar Questions
Assertion (A) Gases do not liquefy above their critical temperature, even on applying high pressure.
Reason (R) Above critical temperature, the molecular speed is high and intermolecular attractions cannot hold the molecules together because they escape because of high speed.
View SolutionA cricketer can throw a ball to a maximum horizontal distance of 150 m. With the same speed how high above the ground can the cricketer throw the ball?
View Solution
Knowledge Check
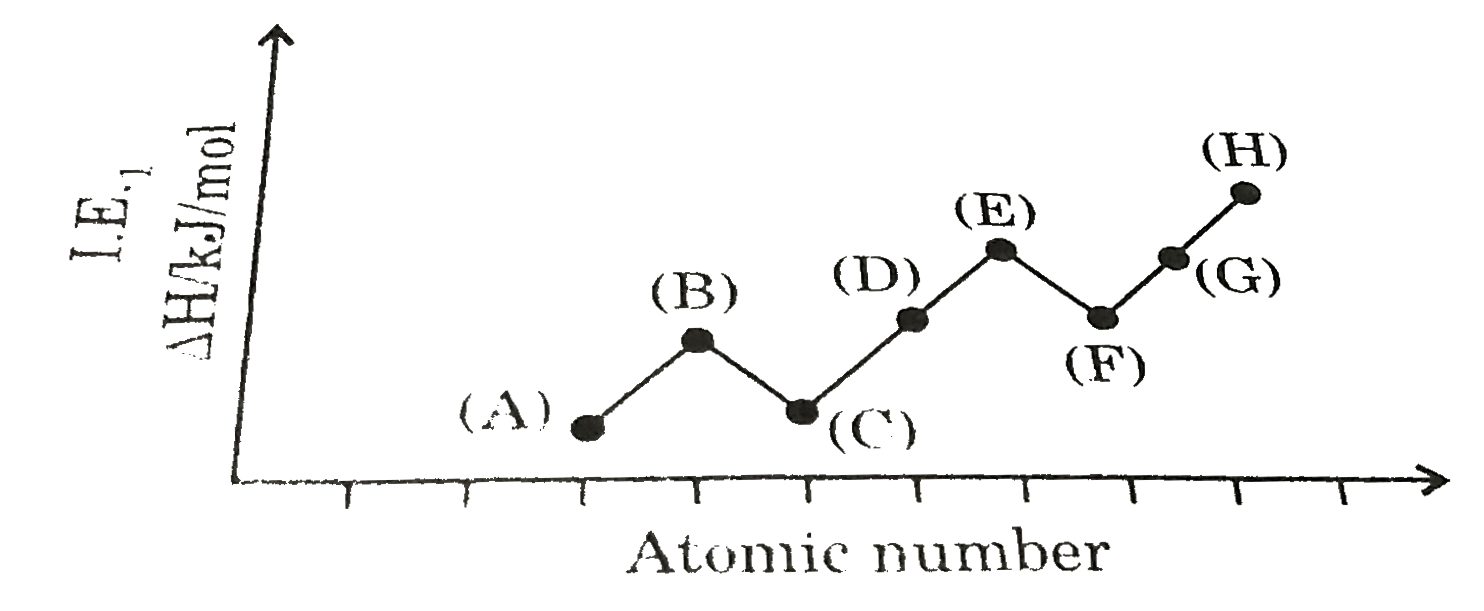
In above graph element F is:AoxygenBnitrogenCfluorineDneonIn the above graph, point D indicates
ALimiting PoointBYield pointCBreaking pointDElastic limitIn the above graph, point B indicates.
ABreaking pointBLimiting pointCYield pointDNone of the above
A cricketer can throw a ball to a maximum horizontal distance of 150 m. With the same speed how high above the ground can the cricketer throw the ball?
View SolutionIn the above graph, point B indicates
View SolutionAssertion (A) Gases do not liquefy above their critical temperature, even on applying high pressure.
Reason (R) Above critical temperature, the molecular speed is high and intermolecular attractions cannot hold the molecules together because they escape because of high speed.
View SolutionAssertion (A) Gases do not liquefy above their critical temperature, even on applying high pressure.
Reason (R) Above critical temperature, the molecular speed is high and intermolecular attractions cannot hold the molecules together because they escape because of high speed.
View SolutionAssertion (A) Gases do not liquefy above their critical temperature, even on applying high pressure.
Reason (R) Above critical temperature, the molecular speed is high and intermolecular attractions cannot hold the molecules together because they escape because of high speed.
View Solution
