Write the four quantum numbers for the differentiating electron of sodium (Na) atom. Solution in Telugu
1) Na (Z = 11)
2) Electronic configuration 1s22s22p63s1
3) The differentiating electron is in 3s orbital.
4) The four quantum numbers are
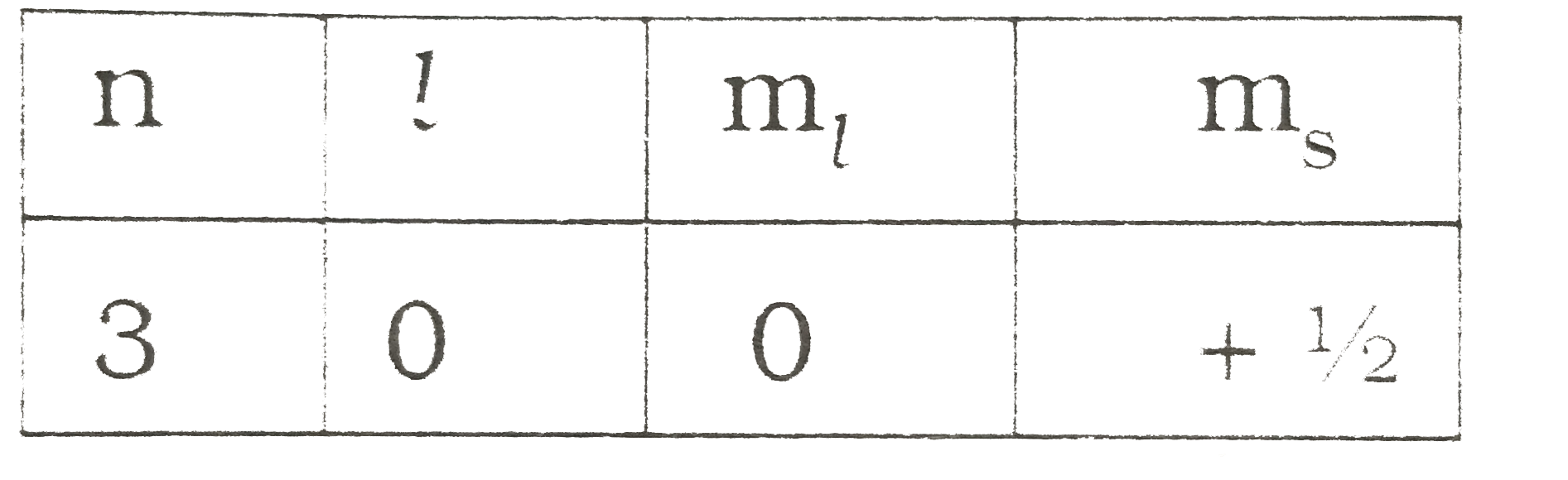
Similar Questions
Write the four quantum numbers of the last electron in the sodium atom /
View SolutionThe horizontal quantum number for the last electron in sodium atom is
View SolutionThe azimuthal quantum number for the last electron in sodium atom is
View SolutionWhich is the correct set of all four quantum number for valance electron of Na ?
View SolutionThe azimuthal quantum number for the last electron in sodium atom is
View SolutionMention the four quantum numbers of the last electron of sodium.
View SolutionAzimuthal quantum number for valence electron of Na atom is________.
View SolutionThe argument of two students is as given : Student 1 : "We need four quantum numbers to represent an electron in a multi- electron atom." Student 2 : " We ned only first three quantum numbers to represent an electron in a multi- electron atom." c) Write the possible four quantum numbers of the valence electron of Na atom.
View Solution
