Write the four quantum numbers for the differentiating electrons of lithium (Li) atom . Solution in Telugu
Video Solution
Text Solution
The electronic configuration of lithium is 1s22s1 . So differentiating electron enters into 2s . The values of four quantum numbers are as given below .
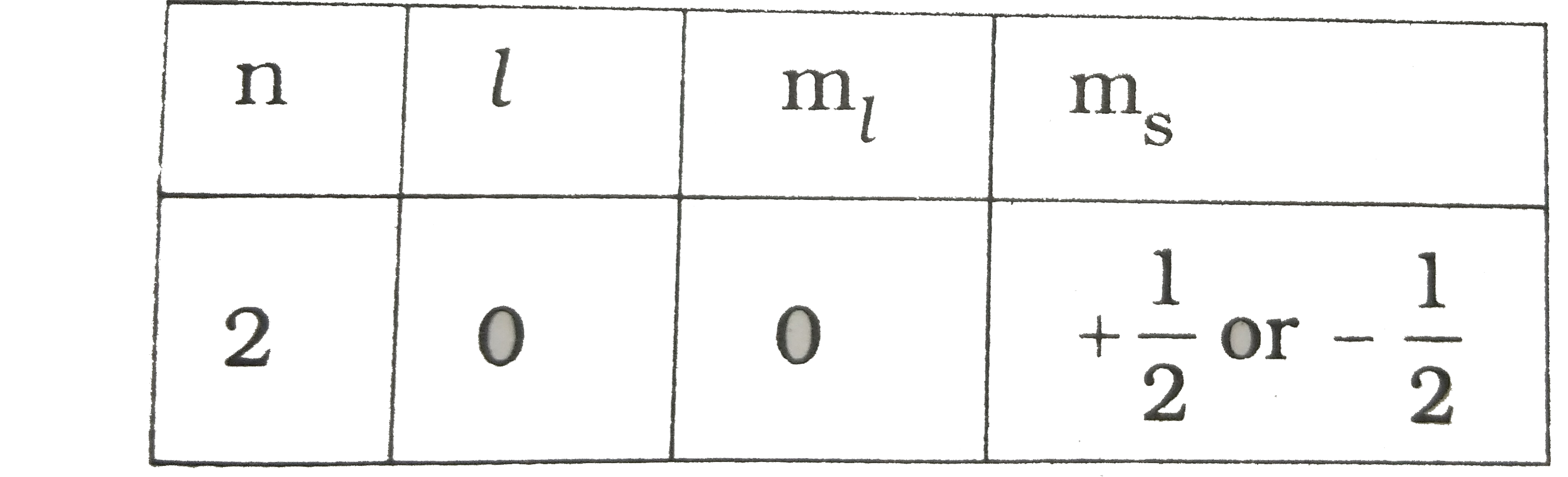
|
Similar Questions
Write the four quantum numbers for the differentiating electron of sodium (Na) atom.
View SolutionWrite four quantum numbers for 1s^(1) electron.
View SolutionWrite four quantum numbers for 2p^(1) electron .
View Solutionदो तत्वों के परमाणु क्रमांक 17 तथा 27 हैं। इनमें प्रविष्ट होने वाले अन्तिम इलेक्ट्रॉनों की चारों क्वाण्टम संख्याएँ लिखिए।
View SolutionWrite down the set of four quantum numbers of the valence shell electron of 3Li.
View SolutionWrite the four quantum numbers of the last electron in the sodium atom /
View SolutionWrite the four quantum numbers for 3d1 electron.
View Solution
