For ideal gas, observation as per Maxwell distribution
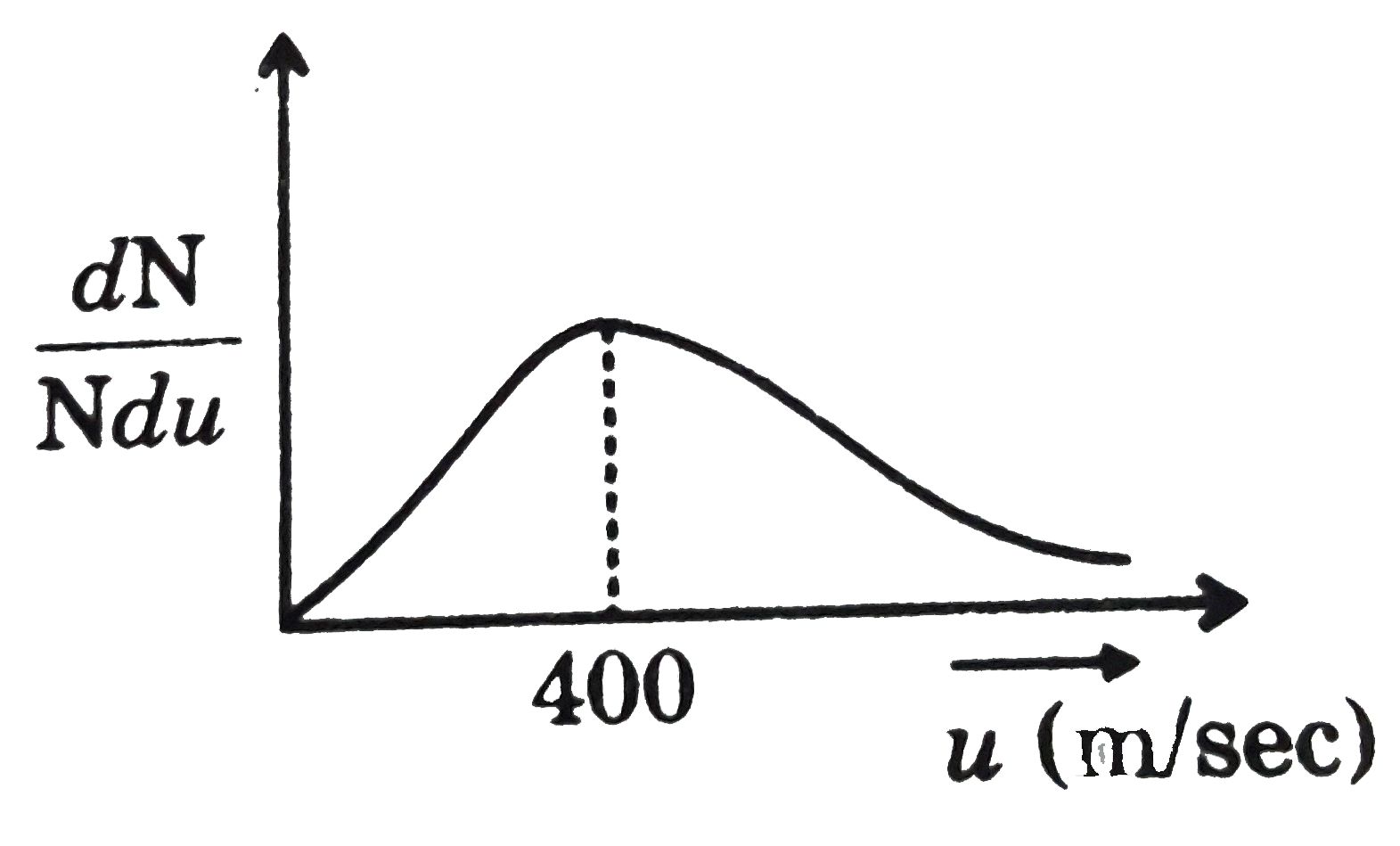
Urms=√1.5×100m/s
Fraction of molecules moving between 400 to 401 m/sec are equal with fraction of molecules moving between 452 to 453 m/sec.
Urms=√20×400m/s
Uavg=1.5Umps
The correct Answer is:A
Step by step video, text & image solution for For ideal gas, observation as per Maxwell distribution by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Maxwell Distribution Curves
View SolutionAn ideal gas consisting of molecules of mass m with concentration n has a temperature T. Using the Maxwell distribution function, find the number of molecules reaching a unit area of a wall at the angles between θ and θ+dθ to its normal per unit time.
View Solution
Knowledge Check
Maxwell distribution is true when
Asystem contain single objectBsystem contains two objectsCsystem is emptyDsystem contains large number of objectsFor an ideal gas, following observation is made as per Maxwell-Boltzmann distrbution. Choose the correct options.
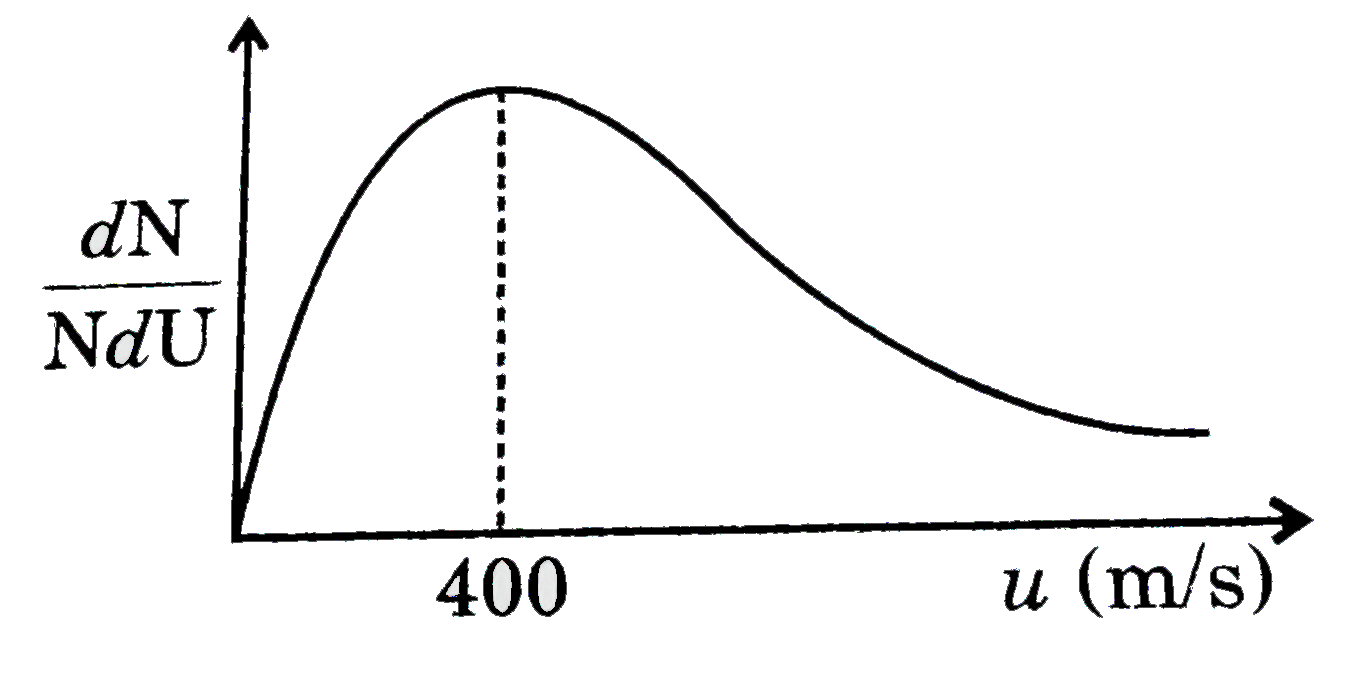 Aμrms=√1.5×400m/sBμavg=√4π×400m/sCFraction of molecules moving between 400 to 401 m/s are more than fraction of molecules moving between 452 to 453 m/sDFraction of molecules moving between 400 to 401 m//s are equal with fraction of molecules moving between 452 to 453 m/s
Aμrms=√1.5×400m/sBμavg=√4π×400m/sCFraction of molecules moving between 400 to 401 m/s are more than fraction of molecules moving between 452 to 453 m/sDFraction of molecules moving between 400 to 401 m//s are equal with fraction of molecules moving between 452 to 453 m/sAssertion : The rms velocity and most probable speeds of the molecules in a gas are same.
The Maxwell distribution curve for the speed of the molecules in a gas is symmetrical.AIf, both Assertion and reason are ture and the Reason is the correct explanation of the Assertion.BIf both, Assertion and reason are true but Reason is not a correct expationation of the Assertion.CIf Assertion is true but the Reason is false.DIf Both, Assertion and reason are false.
Which of the following graphs regarding Maxwell distribution of speed are correct for ideal gases ?
View SolutionFollowing represents the Maxwell distribution curve for an ideal gas at two temperature T1 and T2 . Which of the following option(s) is/are true?
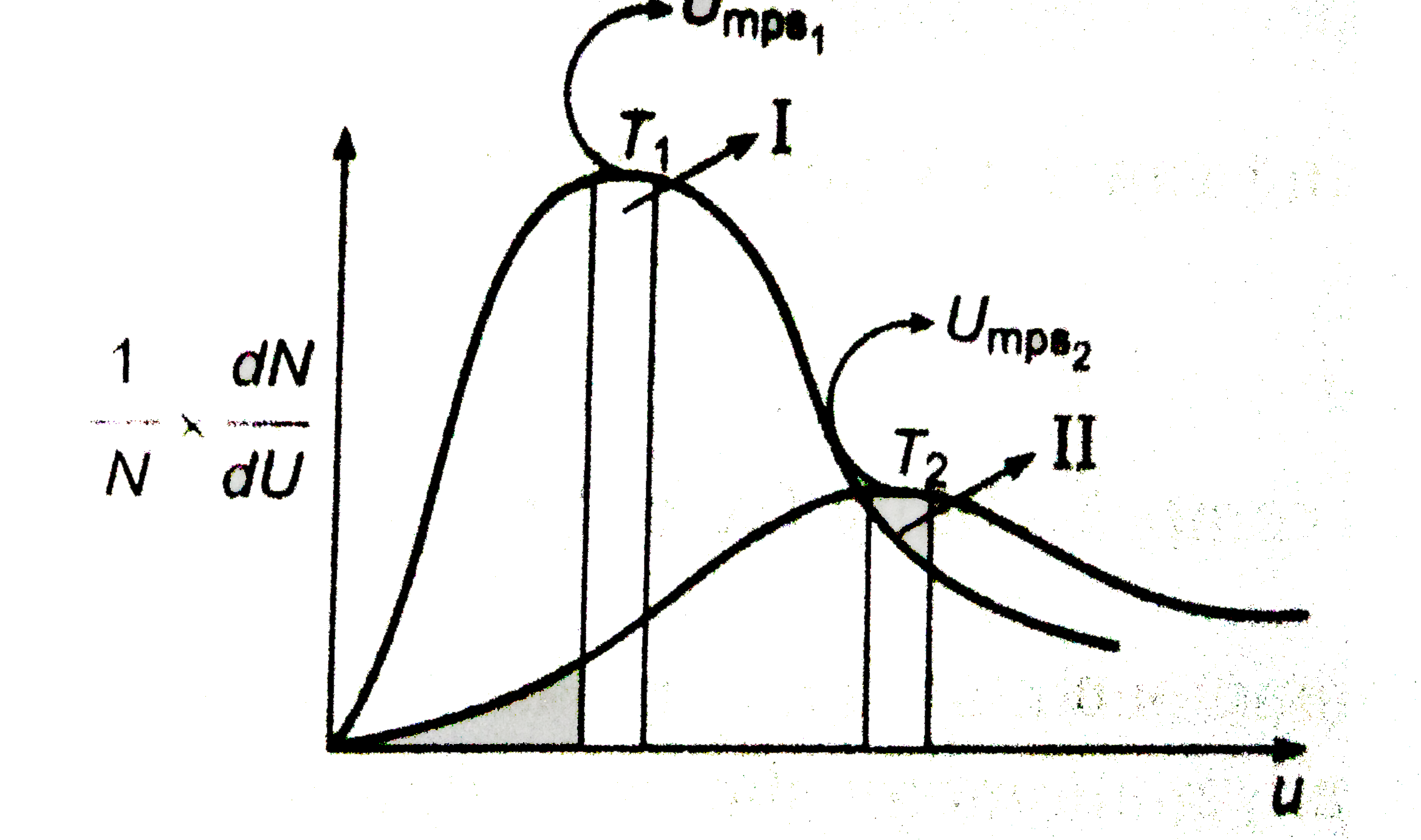
View SolutionFor an ideal gas, number of moles per litre in terms of its pressure P, gas constant R and temperature T is
View SolutionFor an ideal gas, number of moles per litre in terms of its pressure P, gas constant R and temperature T is
View SolutionFollowing are some of the properties of the colloidal sols
I Tyndall effect
brownian motion
III maxwell distribution of molecules
The valid properties are
View Solution
