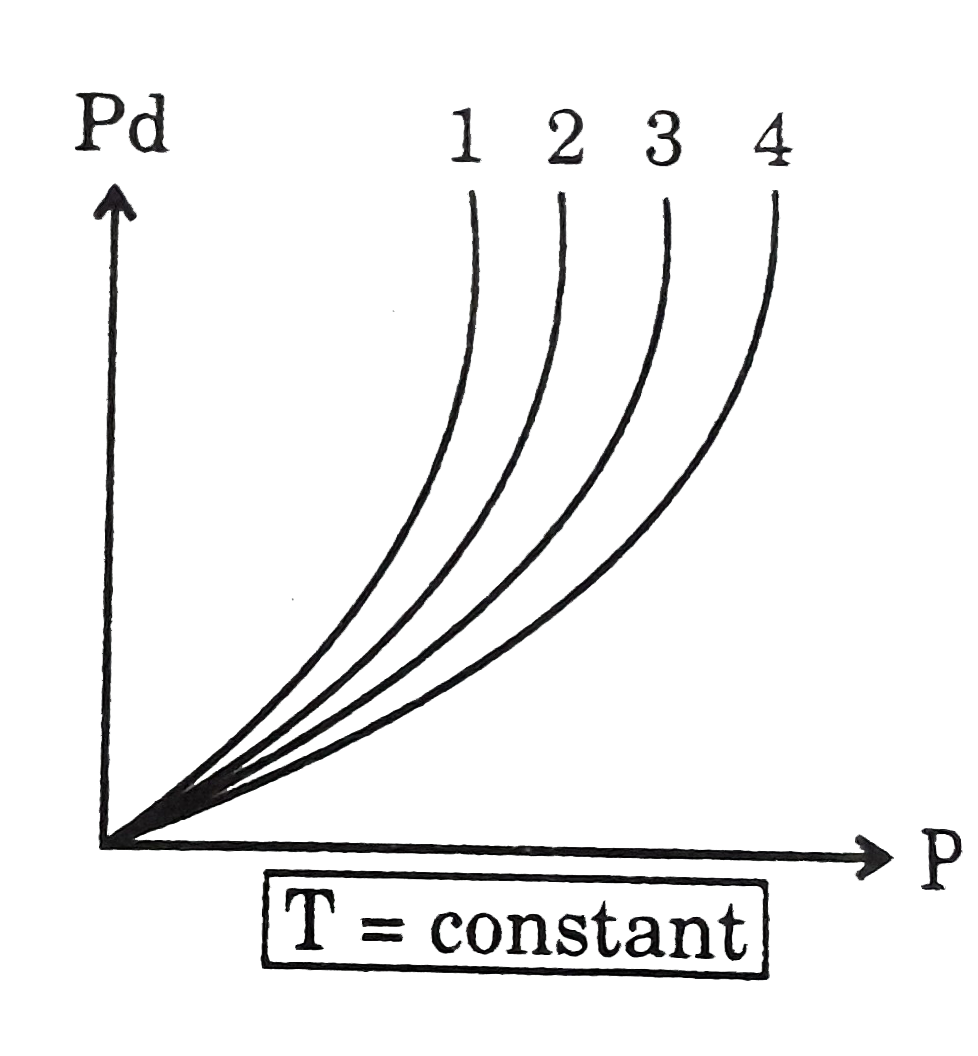
At 273 K, Pd vs P is plotted for various gases 1,2,3,4 assuming ideal behaviour for gases N2,He,CO2 and H2. The correct combination is [P denotes combination in atmosphere and d denotes density in gm/L:
A
2−N2,1−He,3−CO2,4−H2
B
4−N2,1−He,2−CO2,3−H2
C
4−N2,3−He,2−CO2,1−H2
D
2−N2,3−He,1−CO2,4−H2
Text Solution
The correct Answer is:D
|
Similar Questions
Knowledge Check
The volume of gases H2,CH4,CO2 and NH3 absorbed by 1 gm of activated charcoal at 298 K are in the order:
AH2>CH4>CO2>NH3BCH4>CO2>NH3>H2CCO2>NH3>H2>CH4DNH3>CO2>CH4>H2The volume of gases H2,CH4,CO2 and NH3 adsorbed by 1 g of charcoal at 288K are in the order :
AH2,CH4,CO2,NH3BCH4,CO2,NH3,H2CCO2,NH3,H2,CH4DNH3,CO2,CH4,H2The volumes of gases H2,CH4,CO2 and NH3, adsorbed by 1 g charocal at 298 K are in the order .
AH2>CH4>CO2>NH3BCH4>CO2>NH3>H2CCO2>CO2>H2>CH4DNH3>CO2>CH4>H2
Out of CO2 and NH3 gases, which is expectd to show more deviation from the ideal gas behaviour ?
View SolutionExplain the exceptional behaviour of H2 and He on Z-P plot.
View SolutionThe volume of gases H2,CH4,CO2 and NH3 adsorbed by 1 g of charcoal at 288K are in the order :
View SolutionAt constant temperature of 273K,1V vs P are plotted for two ideal gases A and B as given below. Ratio of number of moles of A and B are:
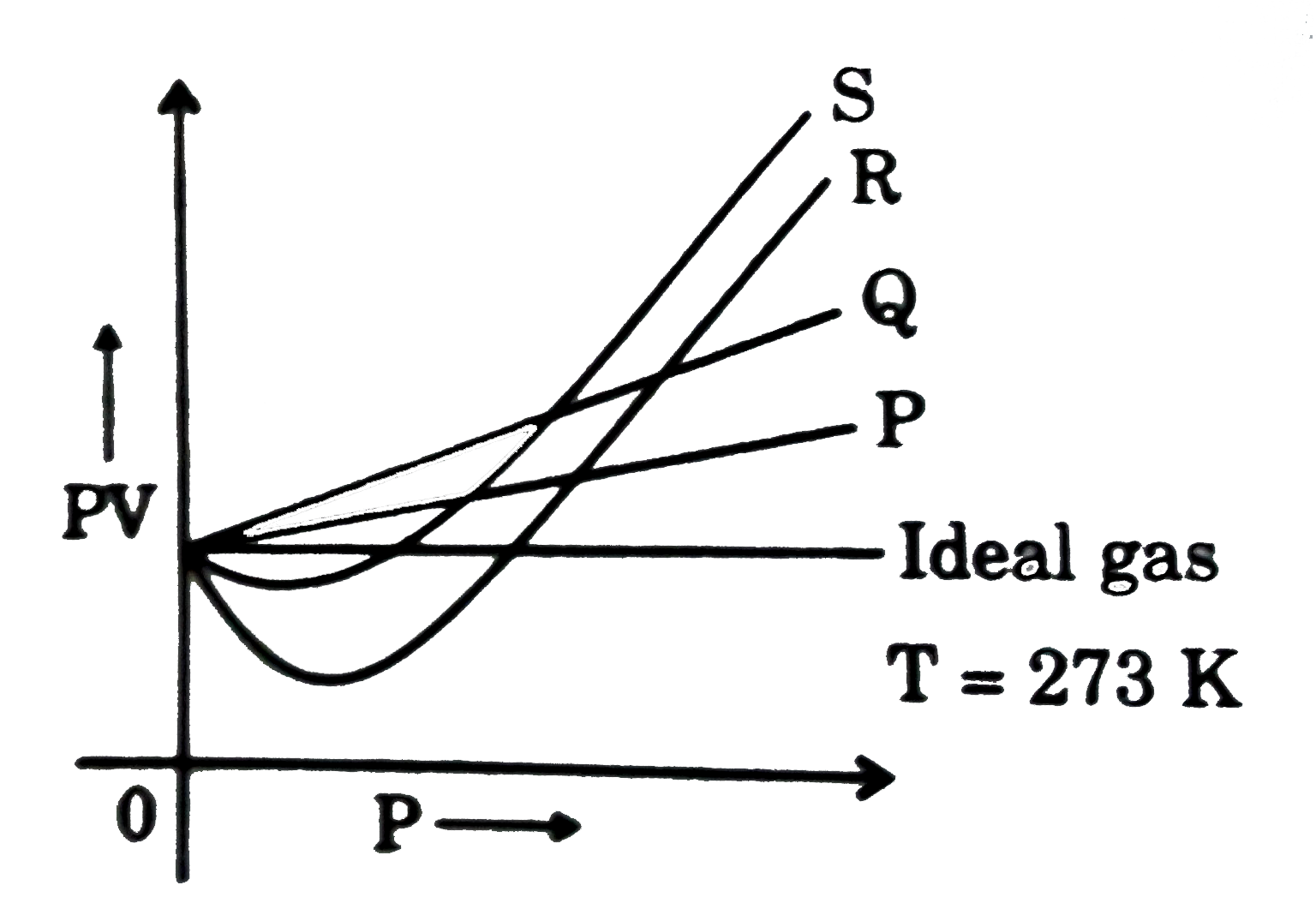
View SolutionFor the given plot, match the most appropriate graph for respective gases: (Among H2,He,CO,CH4)
View Solution

