For the given plot, match the most appropriate graph for respective gases: (Among H2,He,CO,CH4)
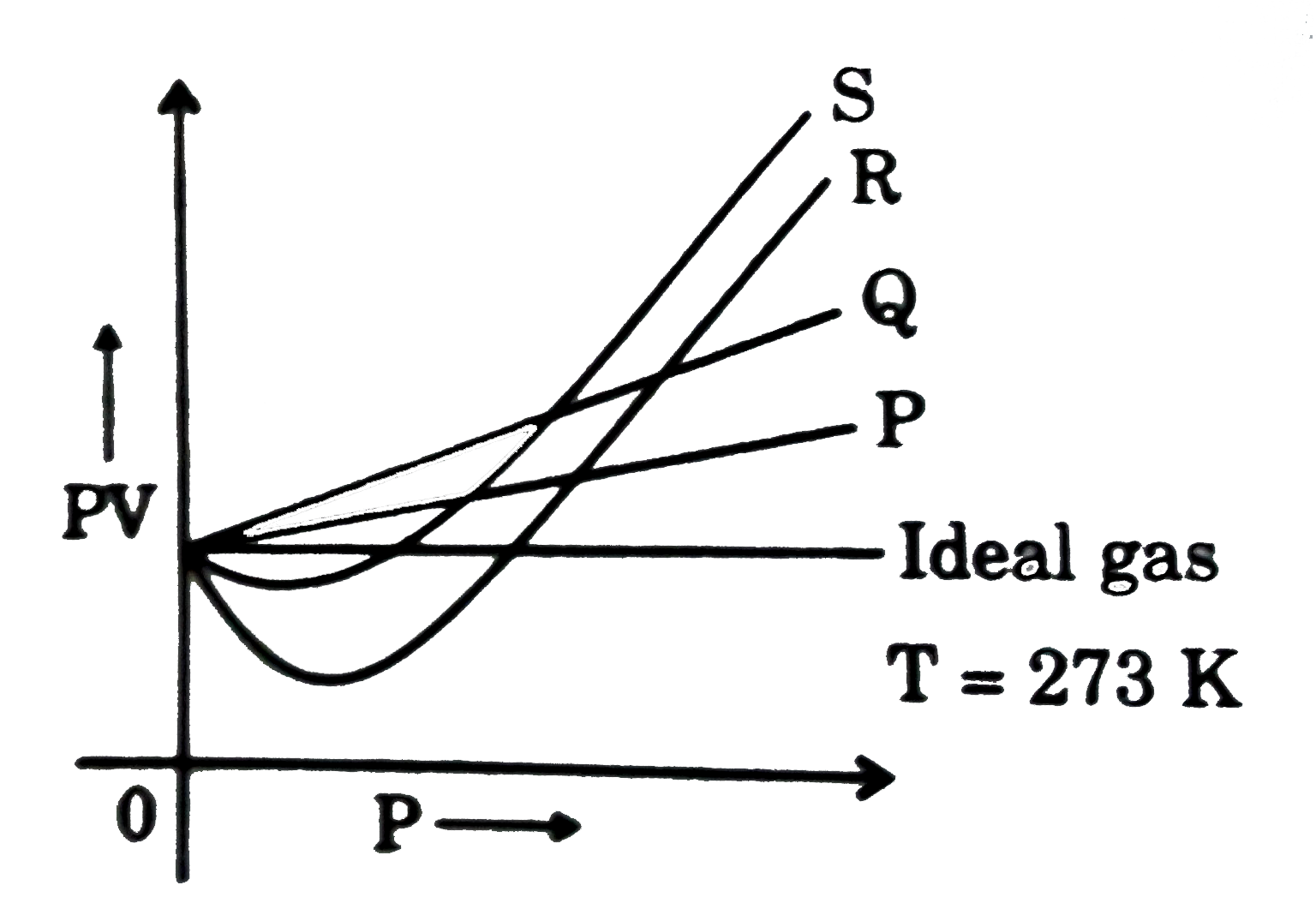
A
PQRSH2HeCOCH4
B
PQRSHeH2CH4CO
C
PQRSHeH2CH4CO
D
PQRSCOHeH2CH4
Text Solution
The correct Answer is:C
|
Similar Questions
Knowledge Check
Choose the most appropriate options.
Among [Ni(CN)4]2−,[NiCl4]2−and[Ni(CO)4]A[NiC4]2− is square planar and [Ni(CN)4]2−Ni(CO)4 are tetrahedralBNi(CO)4 is square planar and [Ni(CN)4]2−[NiCl4]2− are tetrahedralCNi(CN4)2− s square planar [NiCl4]2−Ni(CO)4DNone of theseChoose the most appropriate option.
Consider the following reaction sequence of alkene.
CH3CH=CHCH3O3−−→AH2O−−→znBACH3CH2COCH3BCH3COCH2CCH3CHODCH3CH2CHOThe increasing order of effusion among the gases H2,O2,NH3 and CO2 is
AH2,CO2,NH3,O2BH2,NH3,O2,CO2CH2,O2,NH3,CO2DCO2,O2,NH3,H2
The increasing order of effusion among the gases H2,O2,NH3 and CO2 is
View SolutionIf 1 g of each of the following gases are takes at STP, which of the gases will occupy (a) greatest volume and (b) smallest volume ?
Co,H2O,CH4,NO
View SolutionIf 1 g of each of the following gases are takes at STP, which of the gases will occupy (a) greatest volume and (b) smallest volume ?
Co,H2O,CH4,NO
View SolutionAt 273 K, Pd vs P is plotted for various gases 1,2,3,4 assuming ideal behaviour for gases N2,He,CO2 and H2. The correct combination is [P denotes combination in atmosphere and d denotes density in gm/L:
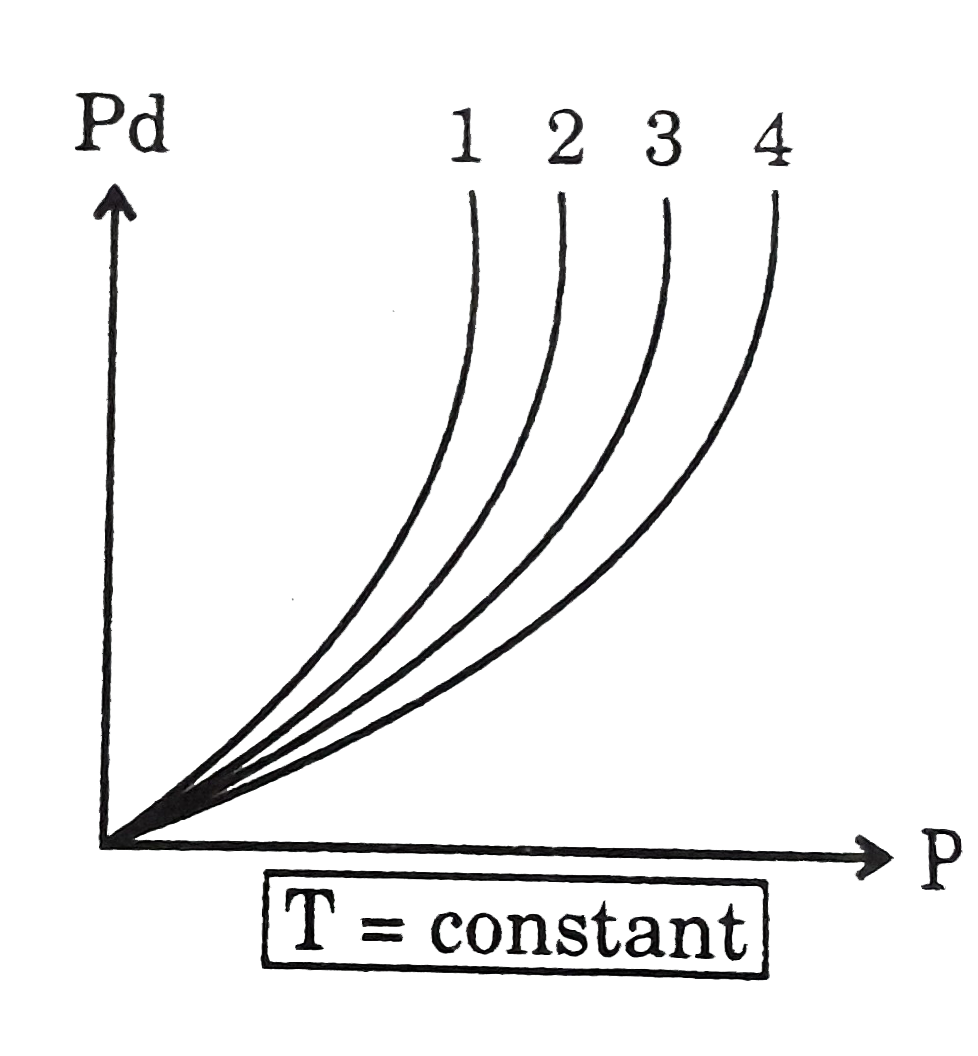
View SolutionThe plot of pV versus p is a straight line for …….. At 273K.
(i) H2
(ii) CO
(iii) CH4
(iv) He
View Solution

