For a 1st order reaction, nA→B whose concentration vs time curve is shown ahead. If half life for the reaction is 24 minutes, the value of n is:
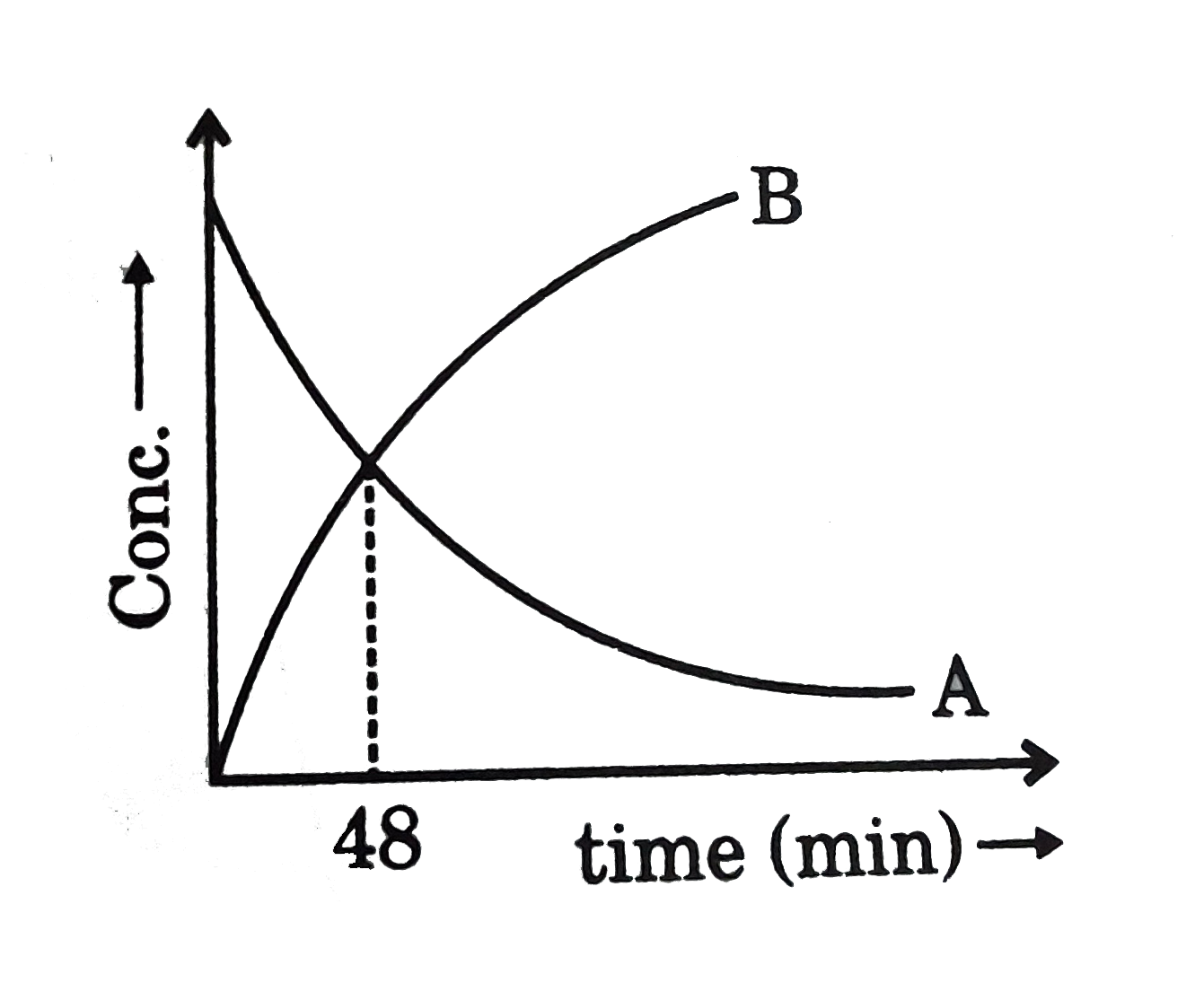
1
2
3
4
The correct Answer is:C
Step by step video, text & image solution for For a 1st order reaction, nA rarr B whose concentration vs time curve is shown ahead. If half life for the reaction is 24 minutes, the value of n is: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
For a first order reaction , nA→B whose concentration vs time curve is as shown in the figure. If half -life for this reaction is 24 minutes. Find out the value of n .
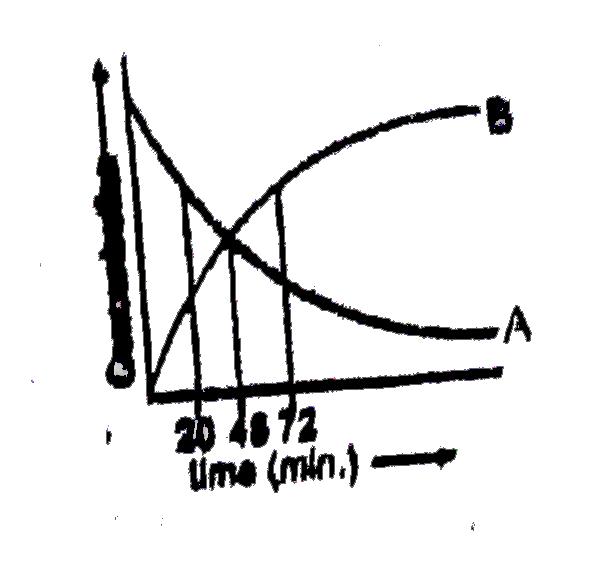 A1B2C3D4
A1B2C3D4In a 1st order reaction, reactant concentration C varies with time t as:
A1/C increases linearly with tBlog C decreases linearly with tCC decreases with 1/tDlog C decreases with 1/tIn a 1st order reaction, reactant concentration C varies with time t as:
A1/C increases linearly with tBlog C decreases linearly with tCC decreases with 1/tDlog C decreases with 1/t
For a first order reaction, if ‘a’ is the initial concentration of reactant, then the half life time is
View SolutionFor a 1st order reaction if concentration is doubled then rate of reaction becomes
View Solution1/[A] versus time is a straight line, the order of reaction is
View SolutionIn a first order reaction A → B, if k is rate constant and initial concentration of the reactant A is 2 M then the half life of the reaction is:
View SolutionRate of chemical reaction nA→Product, is doubled when the concentration of A is increased four times. If the half-life time of the reaction at any temperature is 16 minutes, then the time required for 75% of the reaction to complete is
View Solution
