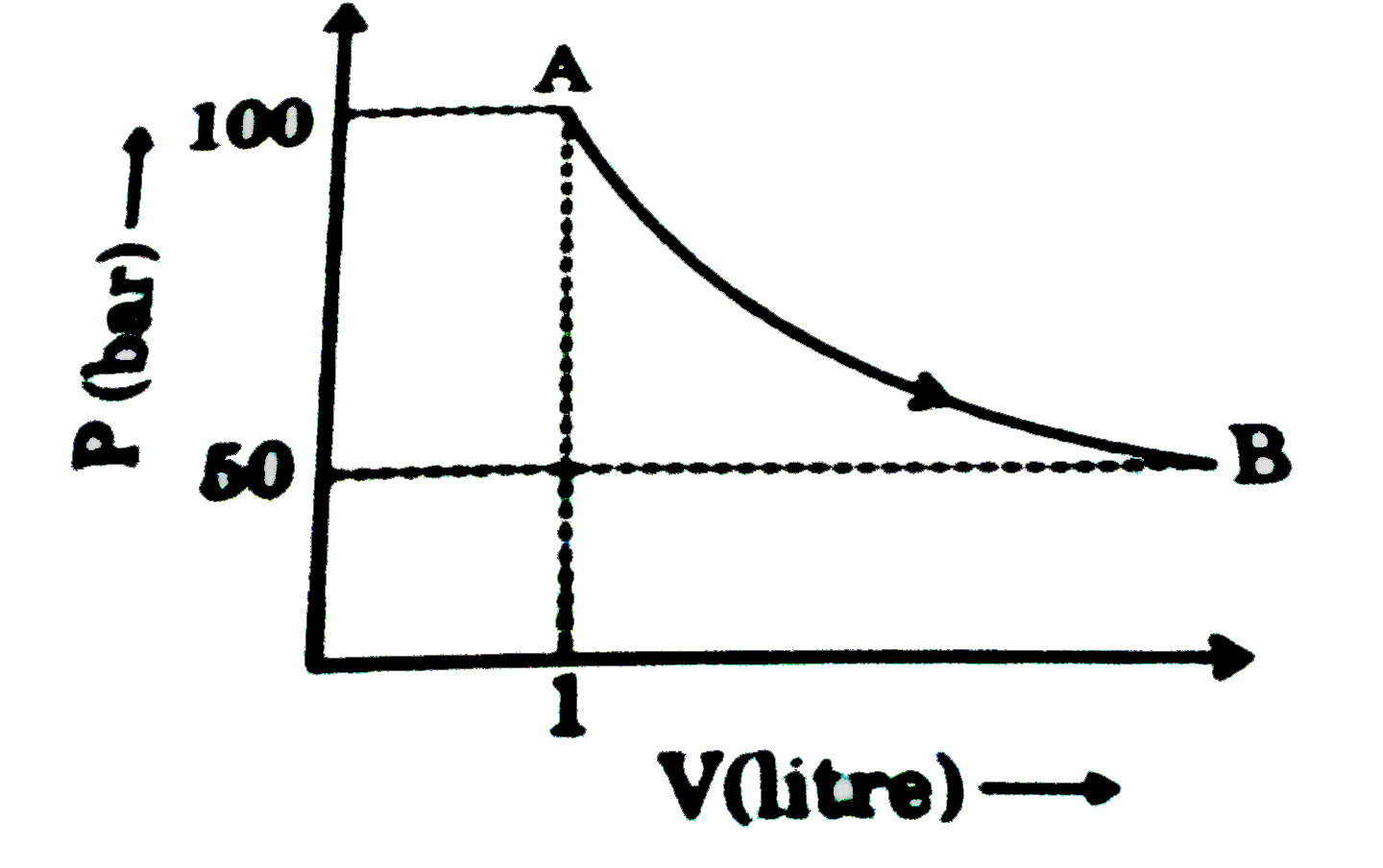
Calculate ΔG (in L atm) from the graph of an ideal gas undergoing process AB. [Take: ln 2 = 0.7]
![Calculate DeltaG (in L atm) from the graph of an ideal gas undergoing process AB. [Take: ln 2 = 0.7]](https://d10lpgp6xz60nq.cloudfront.net/physics_images/GRB_PHY_CHM_P2_V03_QB_C13_E01_423_Q01.png)
The correct Answer is:14
Step by step video, text & image solution for Calculate DeltaG (in L atm) from the graph of an ideal gas undergoing process AB. [Take: ln 2 = 0.7] by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Calculate ΔH (in atm-litre) for a real gas undergoing a change from (10 L, 20 atm) to (50 L,40 atm)
Given : (∂H∂P)v=10litre,(∂H∂V)=5atm
View SolutionCalculate magnitude of ΔH in calorie for 1 mol of an ideal gas undergoing adiabatic reversible process from 8 atm, 300 K to 2 atm. (Given : γ=2,R=2Cal/K/mol)
View Solution
Knowledge Check
For 1 mol of an ideal gas work in process AB will be:
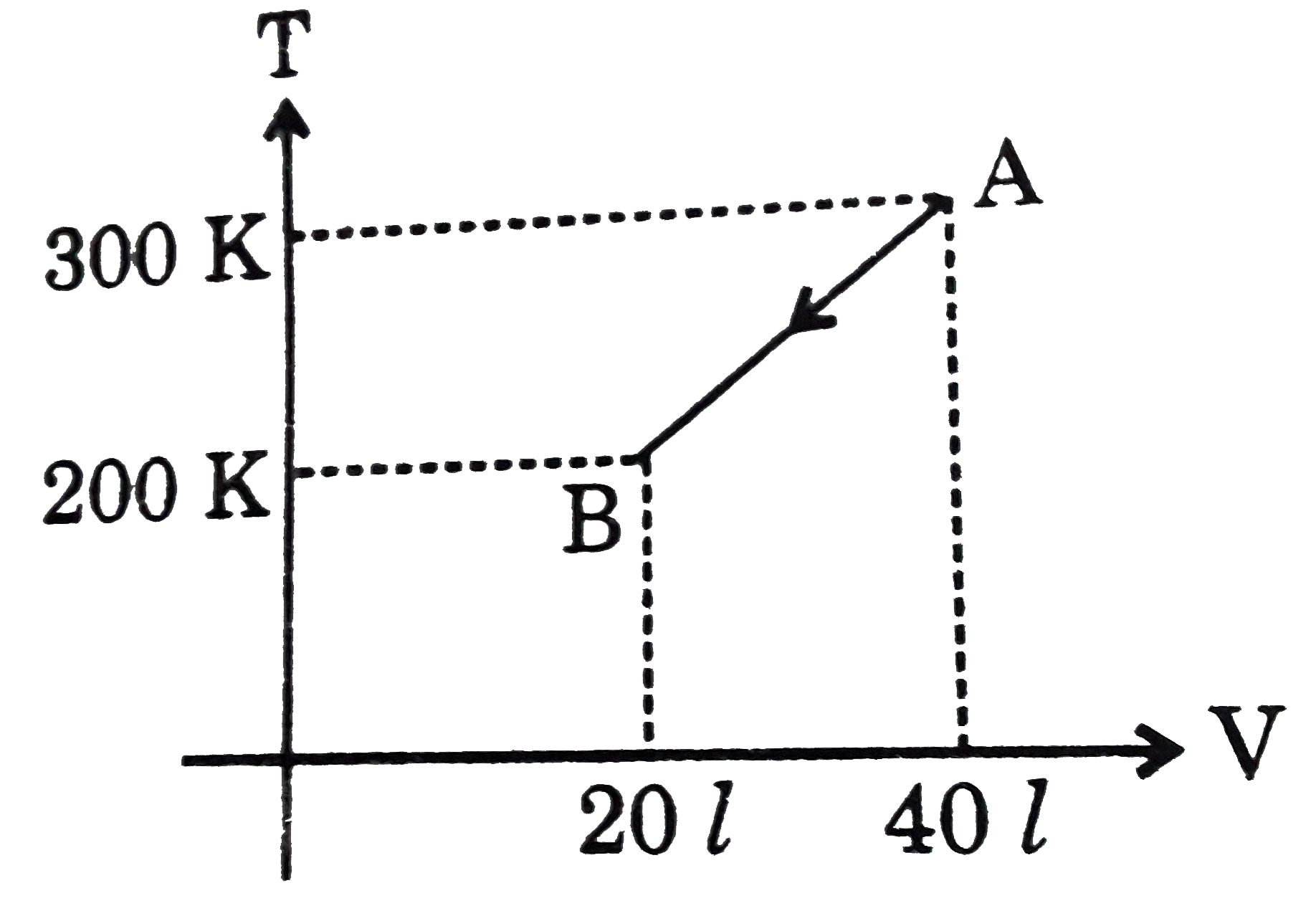 A100RB−100RC100R[1+ln2]D100Rln2
A100RB−100RC100R[1+ln2]D100Rln2During the process AB of an ideal gas
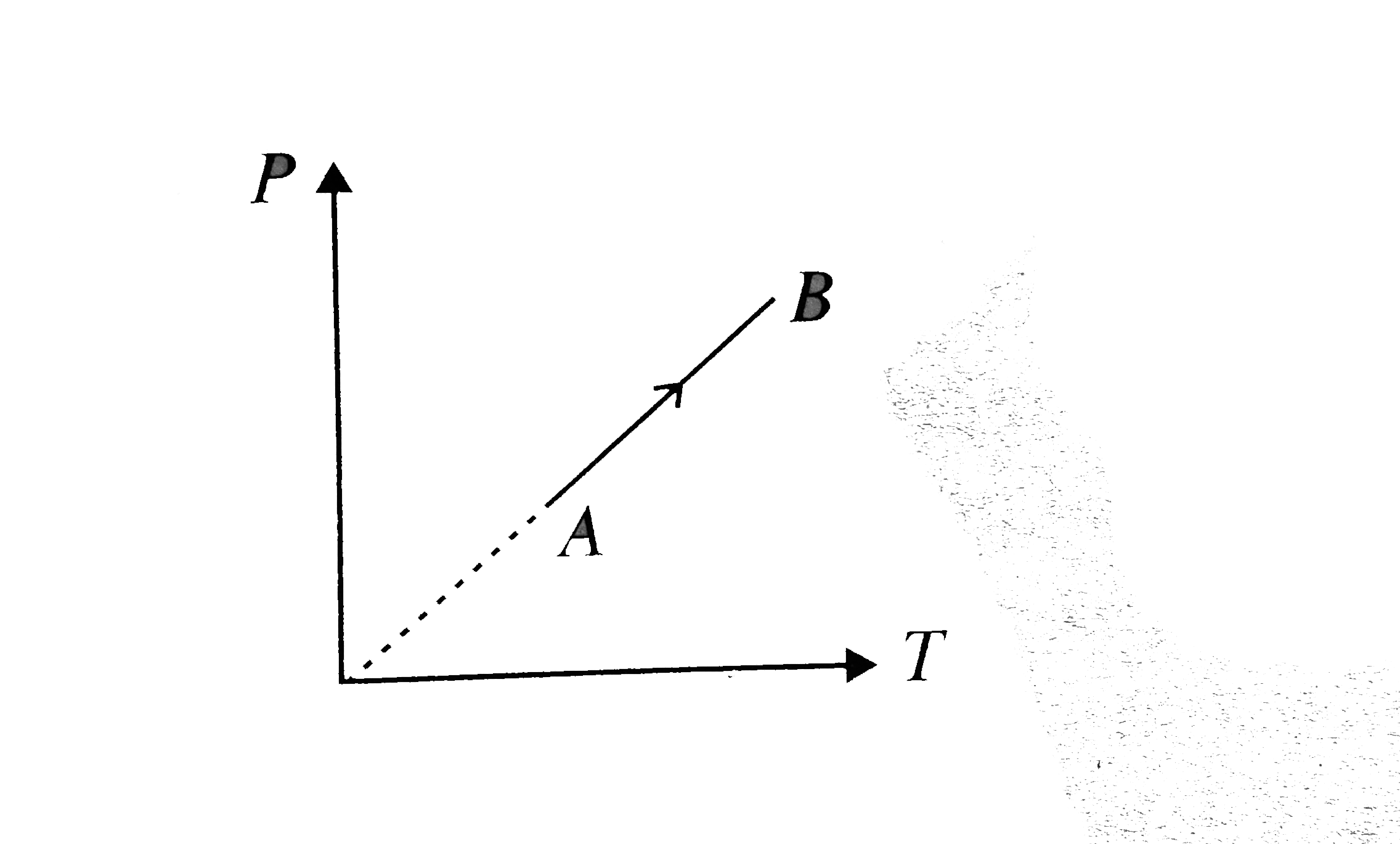 Awork done on the gas is zeroBdensity of the gas is constantCslope of line AB from the T-axis is inversely proportional to the number of moles of the gasDslope of line AB from the T-axis is directly proportional to the number of moles of the gas
Awork done on the gas is zeroBdensity of the gas is constantCslope of line AB from the T-axis is inversely proportional to the number of moles of the gasDslope of line AB from the T-axis is directly proportional to the number of moles of the gasA monoatomic ideal gas undergoes a process ABC. The heat given to the gas is
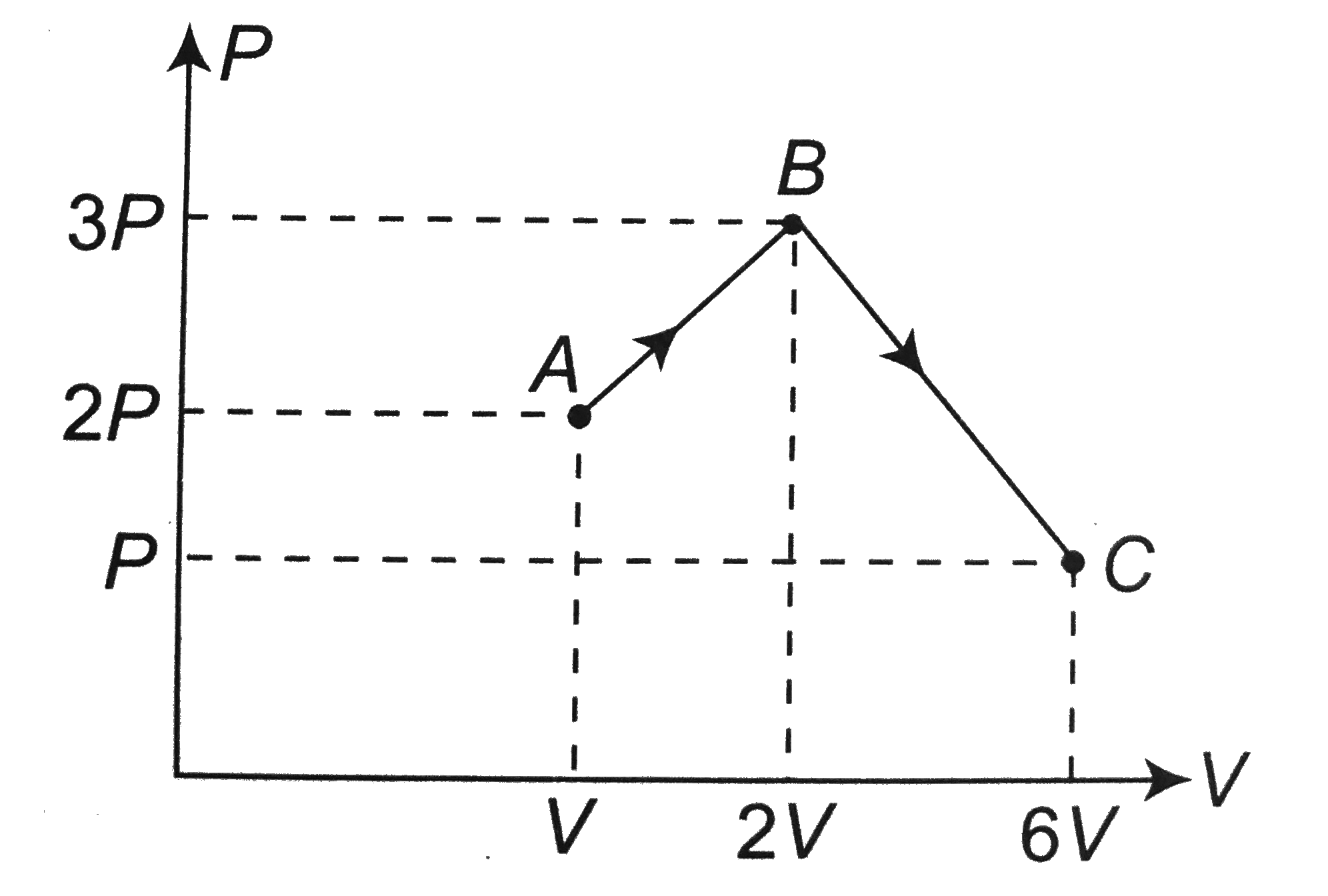 A7.5PVB12.5PVC16.5PVD20.5PV
A7.5PVB12.5PVC16.5PVD20.5PV
Three moles of an ideal gas undergo a cyclic process shown in figure. The work done by the gas during the process is [Take ln (2)=0.693]
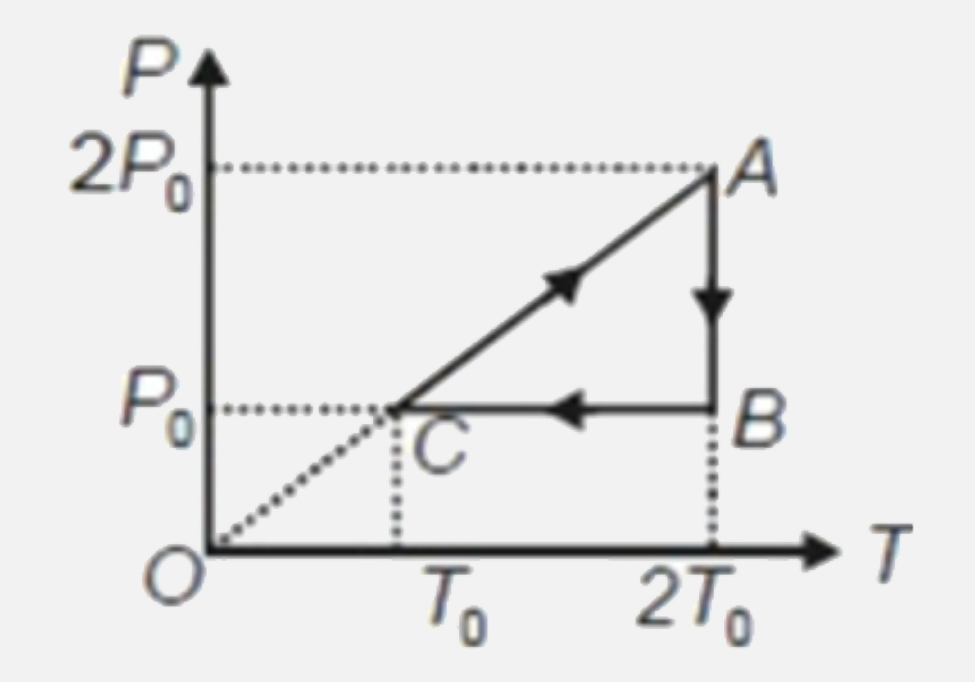
View SolutionCalculate ΔG (in bar-L) when a definite mass of a monoatomic ideal gas at 1 bar and 27∘C is expanded adiabatically against vacuum from 10 L to 20 L (In 2=0.7).
View SolutionFind the heat absorbed by an ideal gas (in kJ)w it follows the graph (given below) during isothermal expansion [ln 2 = 0.7]
[Tale: 1 litre-bar = 0.1 kJ]
View SolutionAn ideal gas undergoes the cyclic process shown in a graph below :
View SolutionTemperature versus pressure graph of an ideal gas is shown in Fig. During the process AB

View Solution
