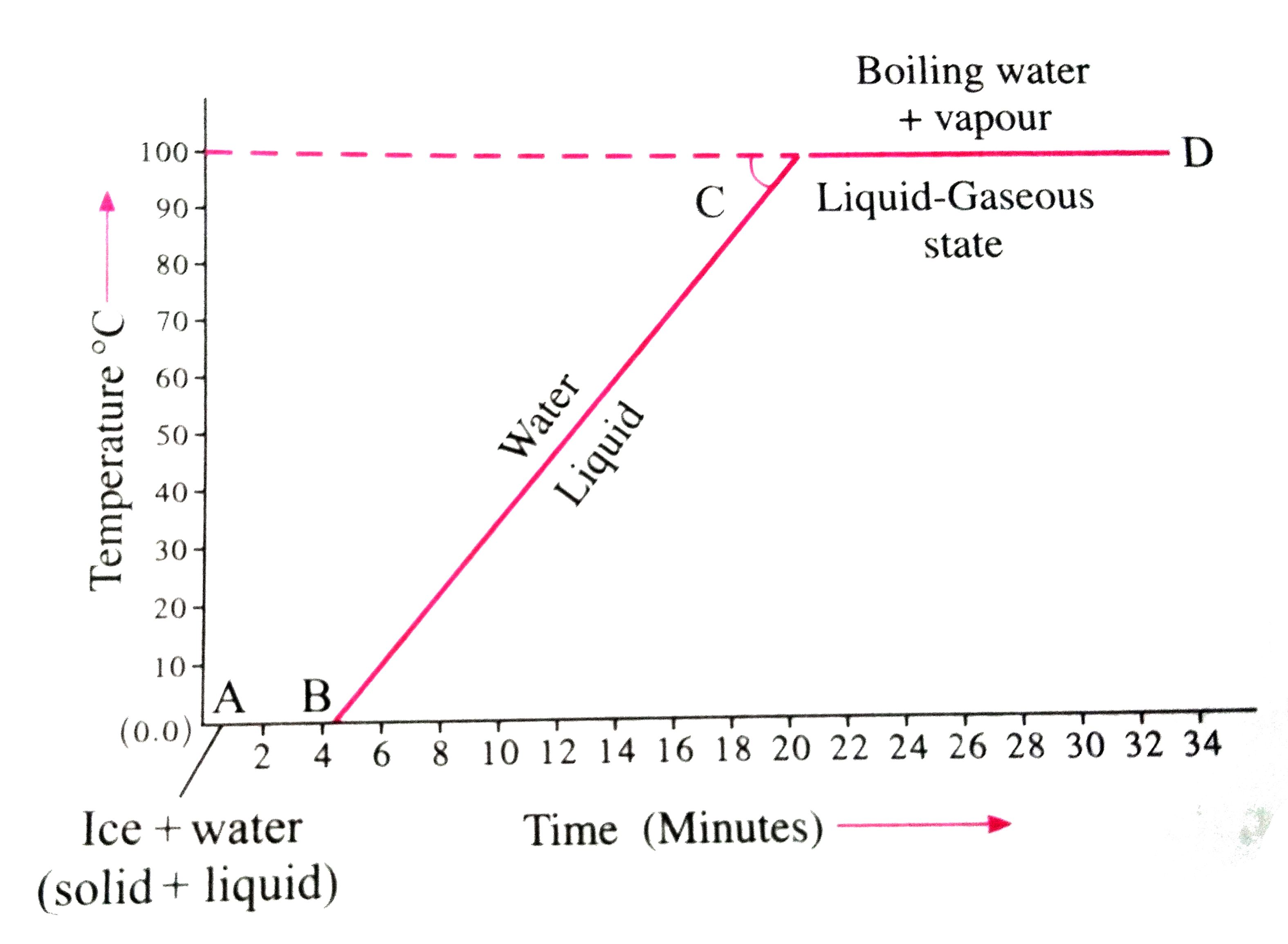
Continue heating even after ice starts melting.
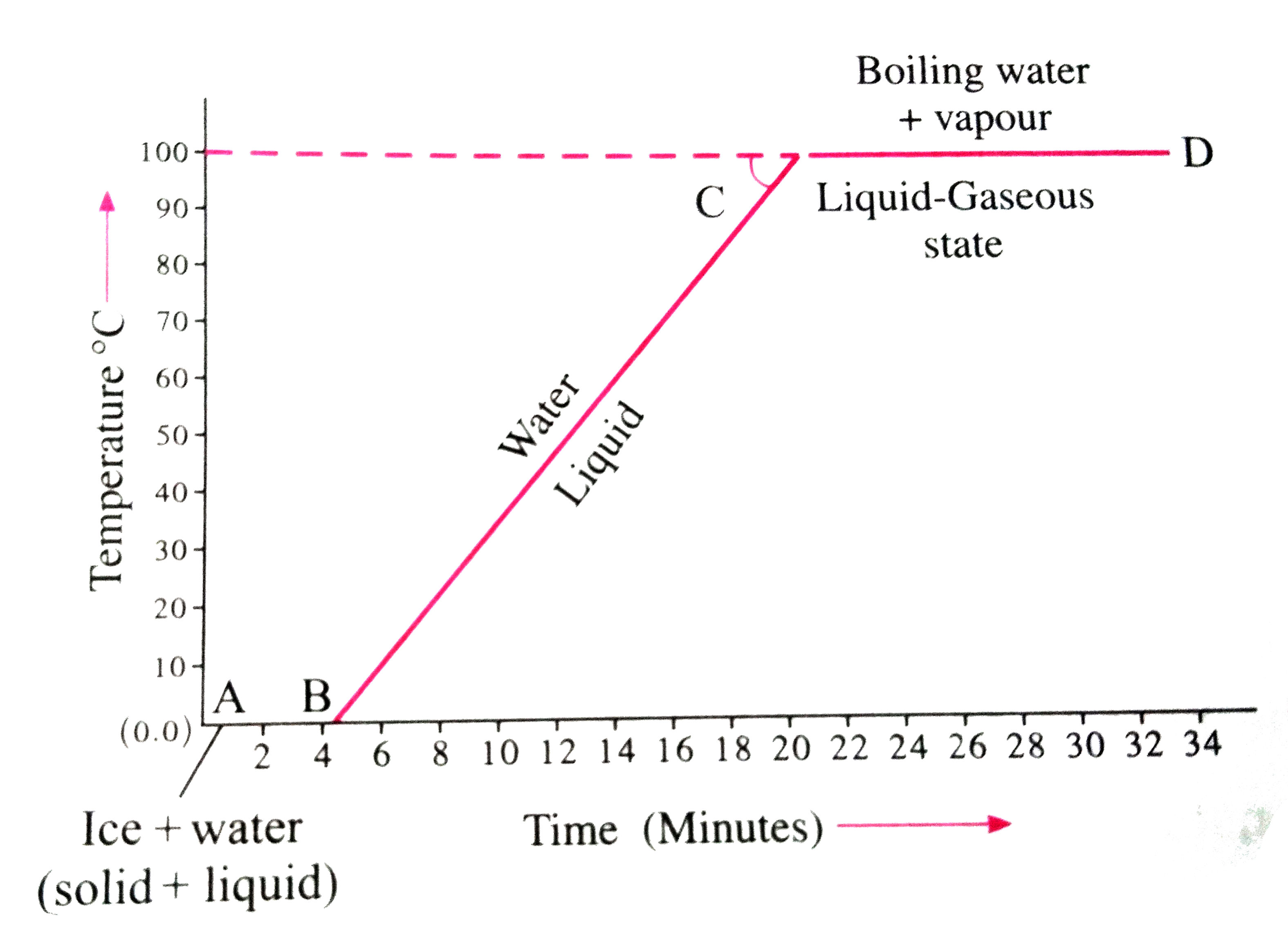
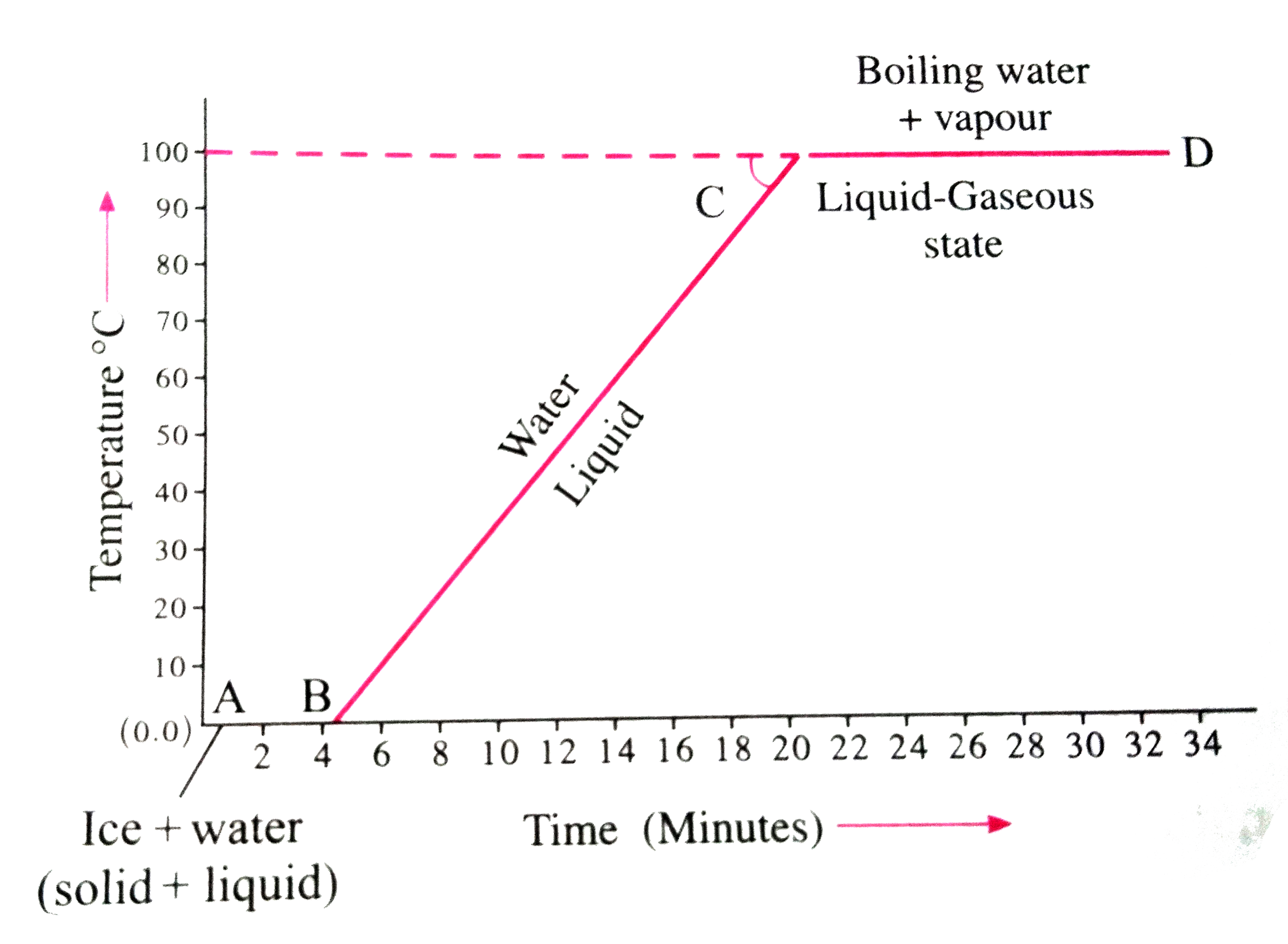
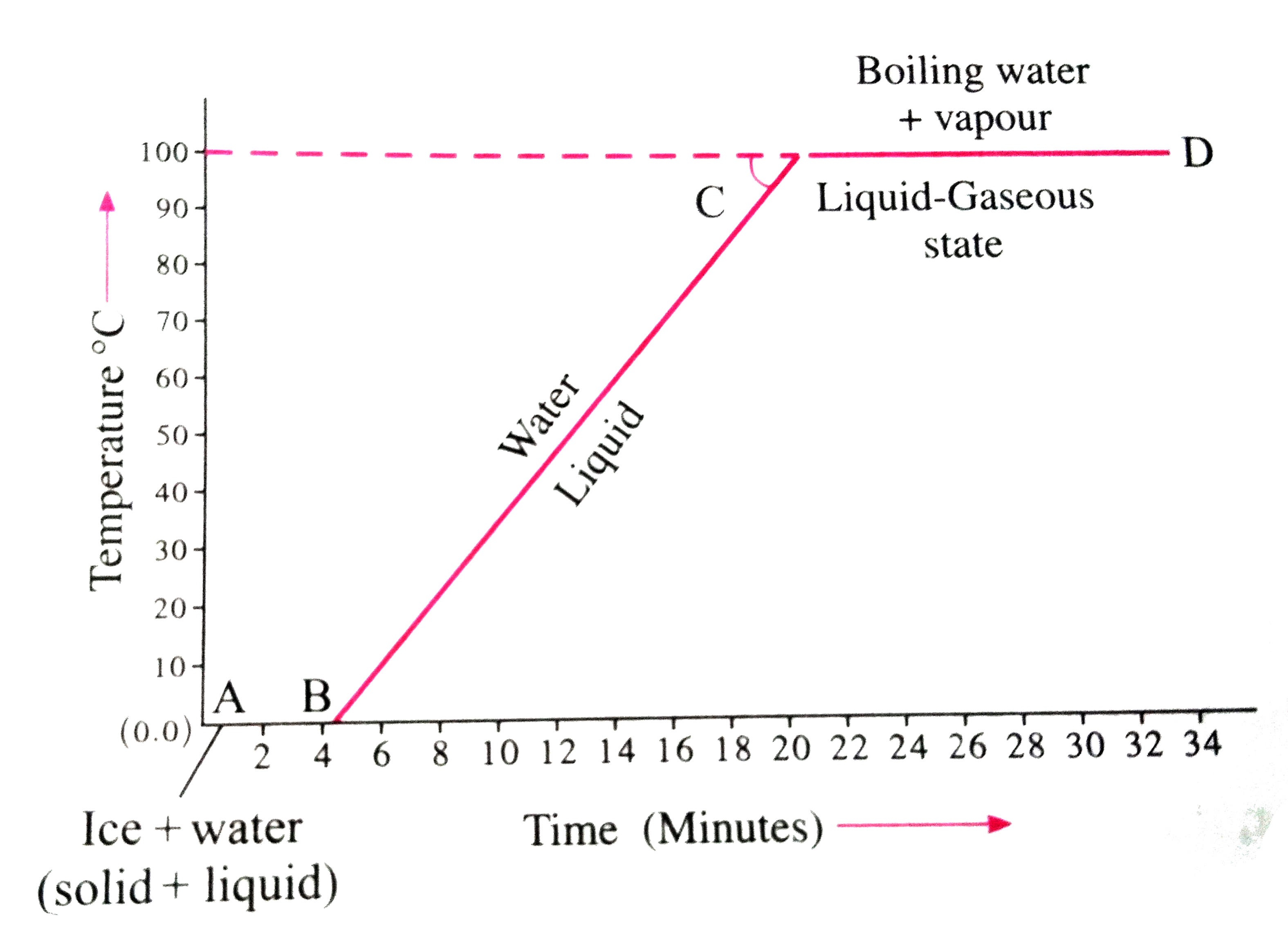
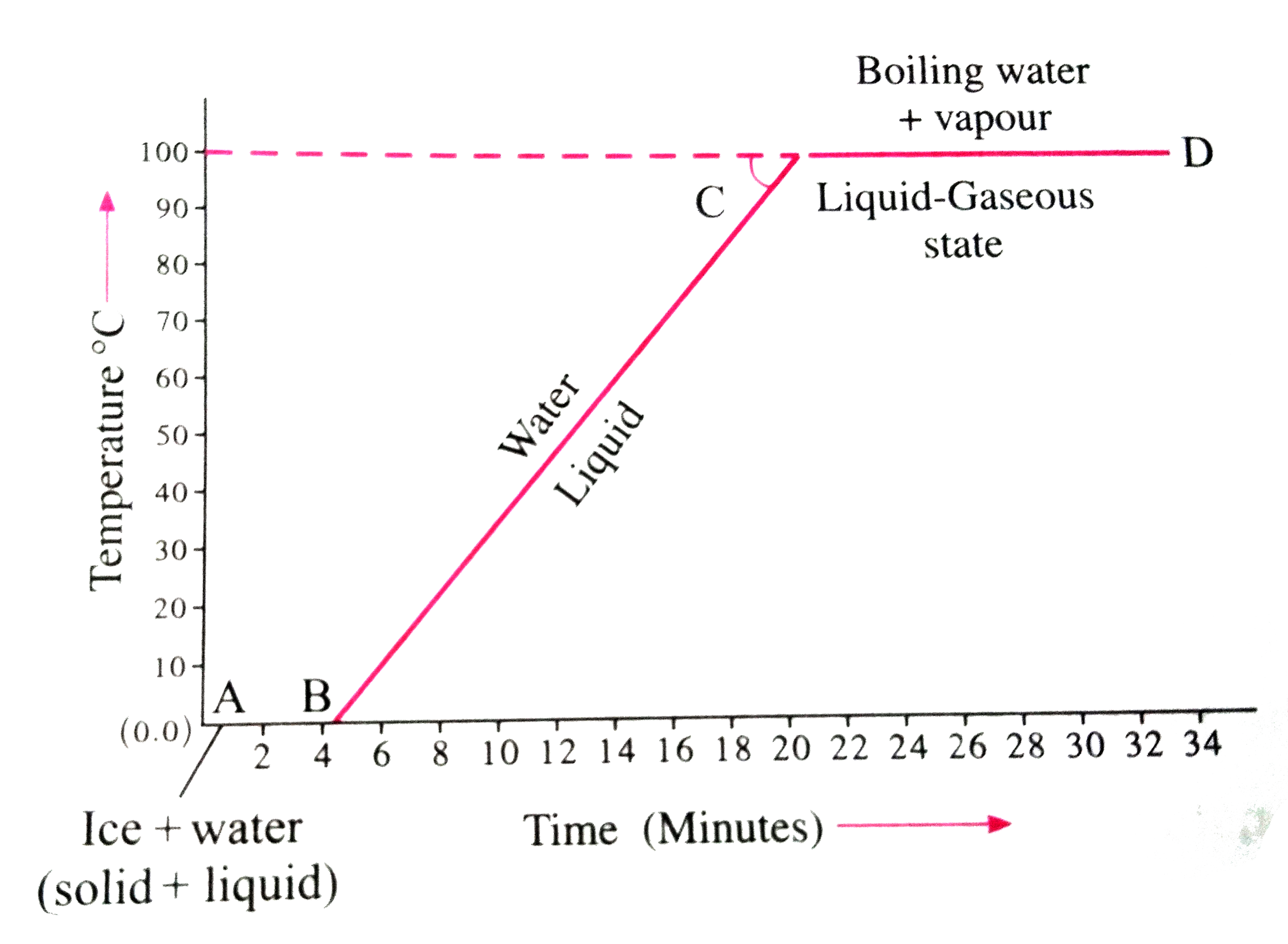
The graph of temperature versus time is shown below.

Topper's Solved these Questions
- HEATBook:TARGET PUBLICATIONChapter:HEATExercise:Chapter Assessment
Explore 18 Videos
- HEATBook:TARGET PUBLICATIONChapter:HEATExercise:Distinguish between
Explore 8 Videos
- HEATBook:TARGET PUBLICATIONChapter:HEATExercise:Practice problems
Explore 20 Videos
- GRAVITATIONBook:TARGET PUBLICATIONChapter:GRAVITATIONExercise:ASSESSMENT
Explore 19 Videos
- LENSES Book:TARGET PUBLICATIONChapter:LENSES Exercise:Chapter Assessment
Explore 21 Videos
Similar Questions
If heating is continued even after ice starts melting then, ______
View SolutionMelting point of solid and boiling point of liquid.
View SolutionHow much heat energy will be given off in cooling water of 2.5 kg from85∘C to 35∘C?
View SolutionAn apple falls from a tree because of gravitational between the earth and apple. If F1 is the magnitude of force exerted by the earth on the apple and F2 is the magnitude of force exerted by apple on earth, then
View SolutionEmma is observing an image of a pencil through a convex lens, if she wants to see the erect image of the pencil she should place the pencil
View SolutionWhy does the temperature remain constant during the melting of ice even though heat is supplied continuously ?
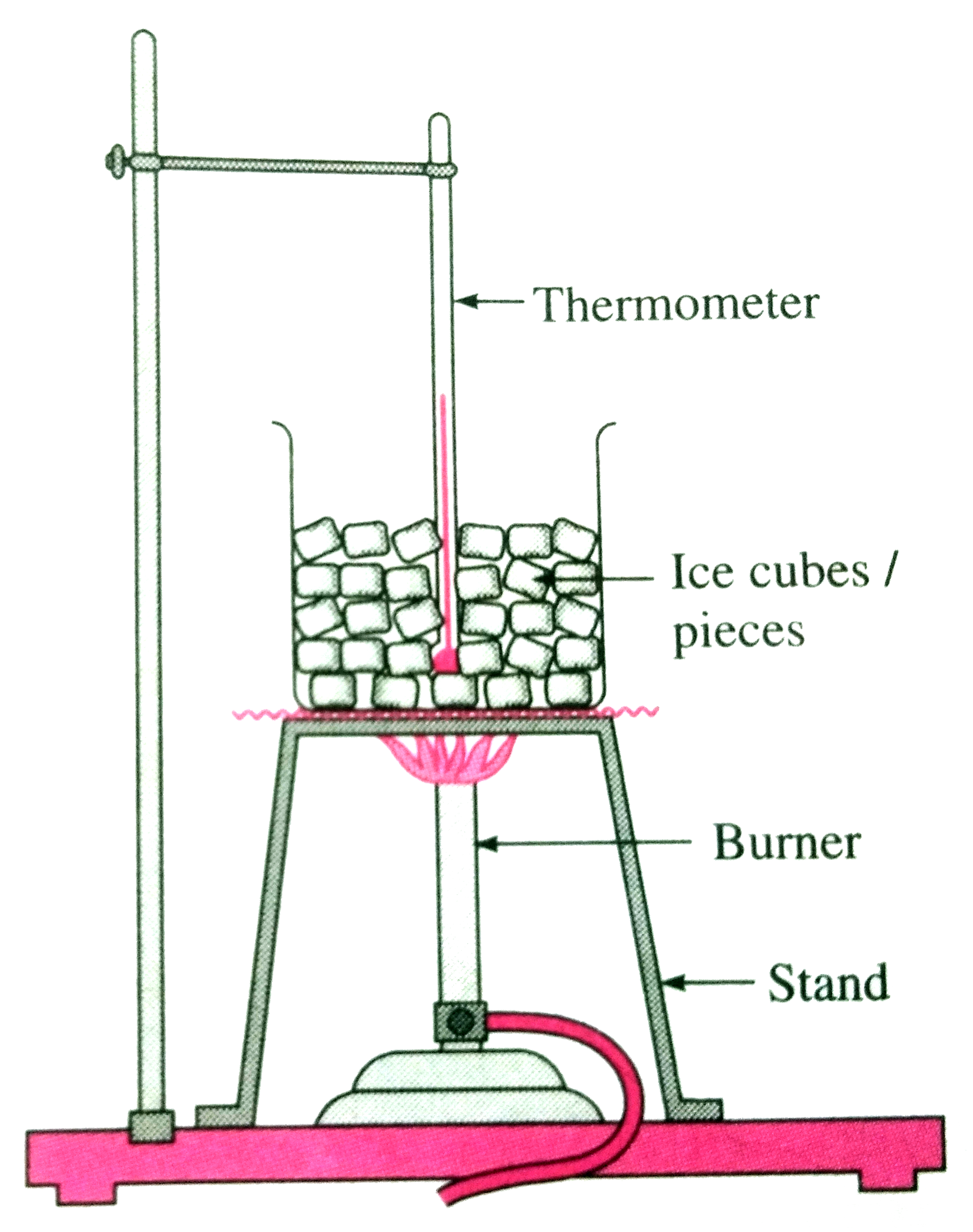
View Solution(1) Take a few pieces of ice in a glass beaker as shown in figure. (2) Insert the bulb of a thermometer in ice and measure its temperature. (3) Put the beaker on a stand and heat the ice usinga burner. (4) Record the temperature using the thermometer after every minute. (5) As the ice is heated,it starts melting. Stir the mixture of ice and water. (6) Continue the heating even after ice starts melting. (7) Draw a graph of temperature versus time. Explain the following temperature vs time graph.
View Solution(1) Take a few pieces of ice in a glass beaker as shown in figure.
(2) Insert the bulb of a thermometer in ice and measure its temperature.
(3) Put the beaker on a stand and heat the ice using a burner.
(4) Record the temperature using the thermometer after every minute.
(5) As the ice is heated,it starts melting. Stir the mixture of ice and water.
(6) Continue the heating even after ice starts melting.
(7) Draw a graph of temperature versus time.
Explain the role of latent heat in the change of state of a substance.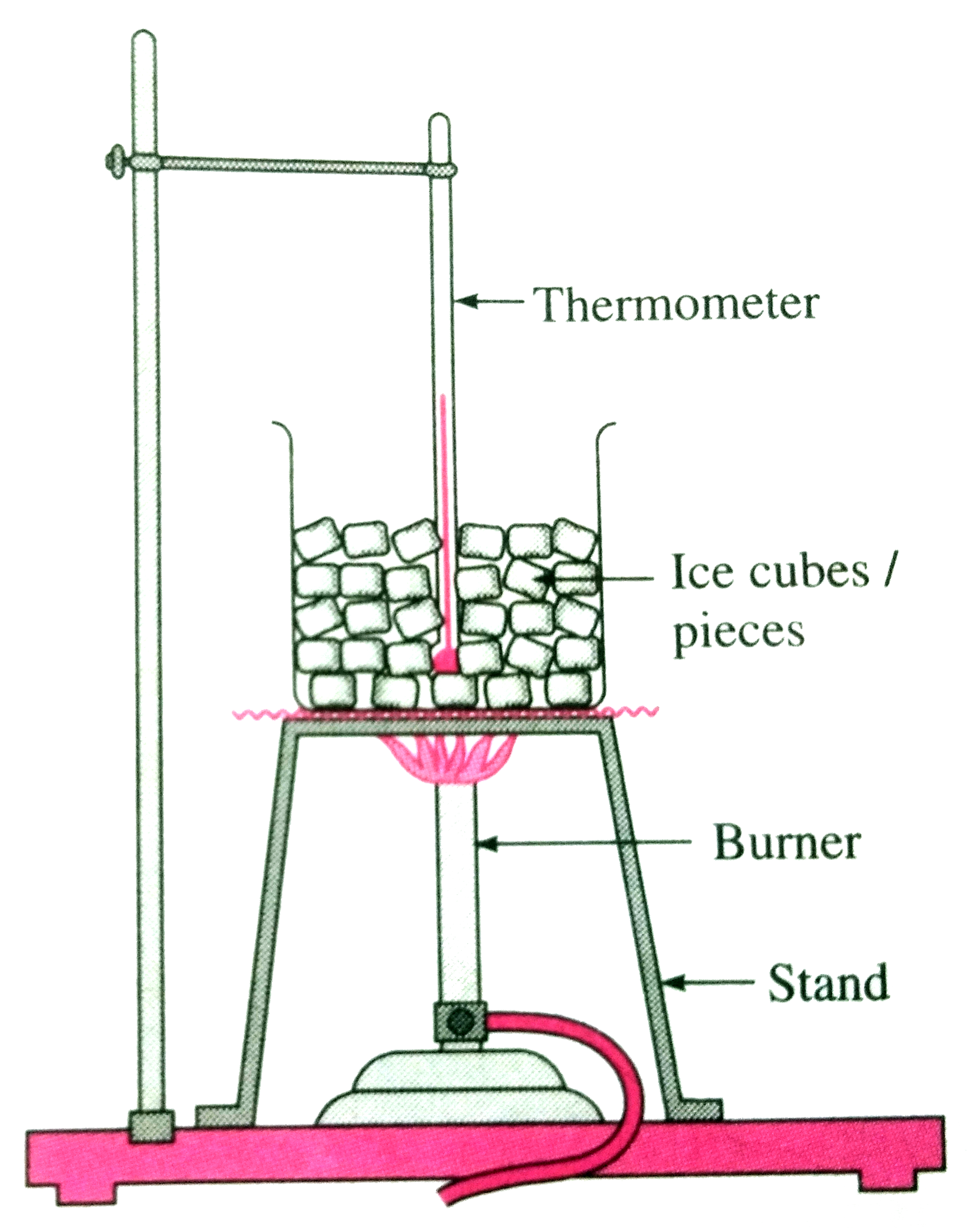
View Solution(1) Take a few pieces of ice in a glass beaker as shown in figure.
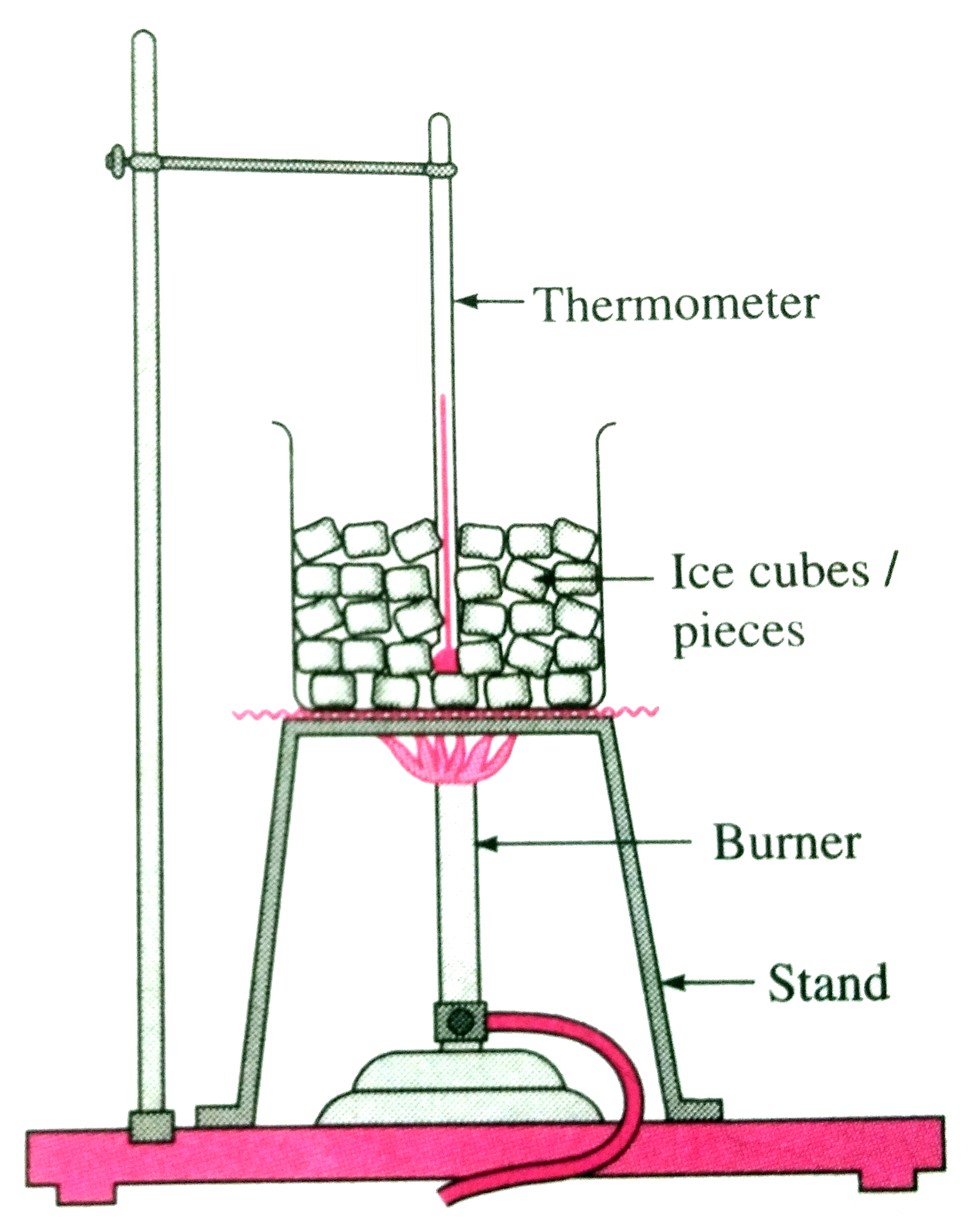
(2) Insert the bulb of a thermometer in ice and measure its temperature.
(3) Put the beaker on a stand and heat the ice using a burner.
(4) Record the temperature using the thermometer after every minute.
(5) As the ice is heated,it starts melting. Stir the mixture of ice and water.
(6) Continue the heating even after ice starts melting.
(7) Draw a graph of temperature versus time.
What is meant by latent heat ?How will the state of matter transform if latent heat is given off ?
View Solution(1) Take a few pieces of ice in a glass beaker as shown in figure.
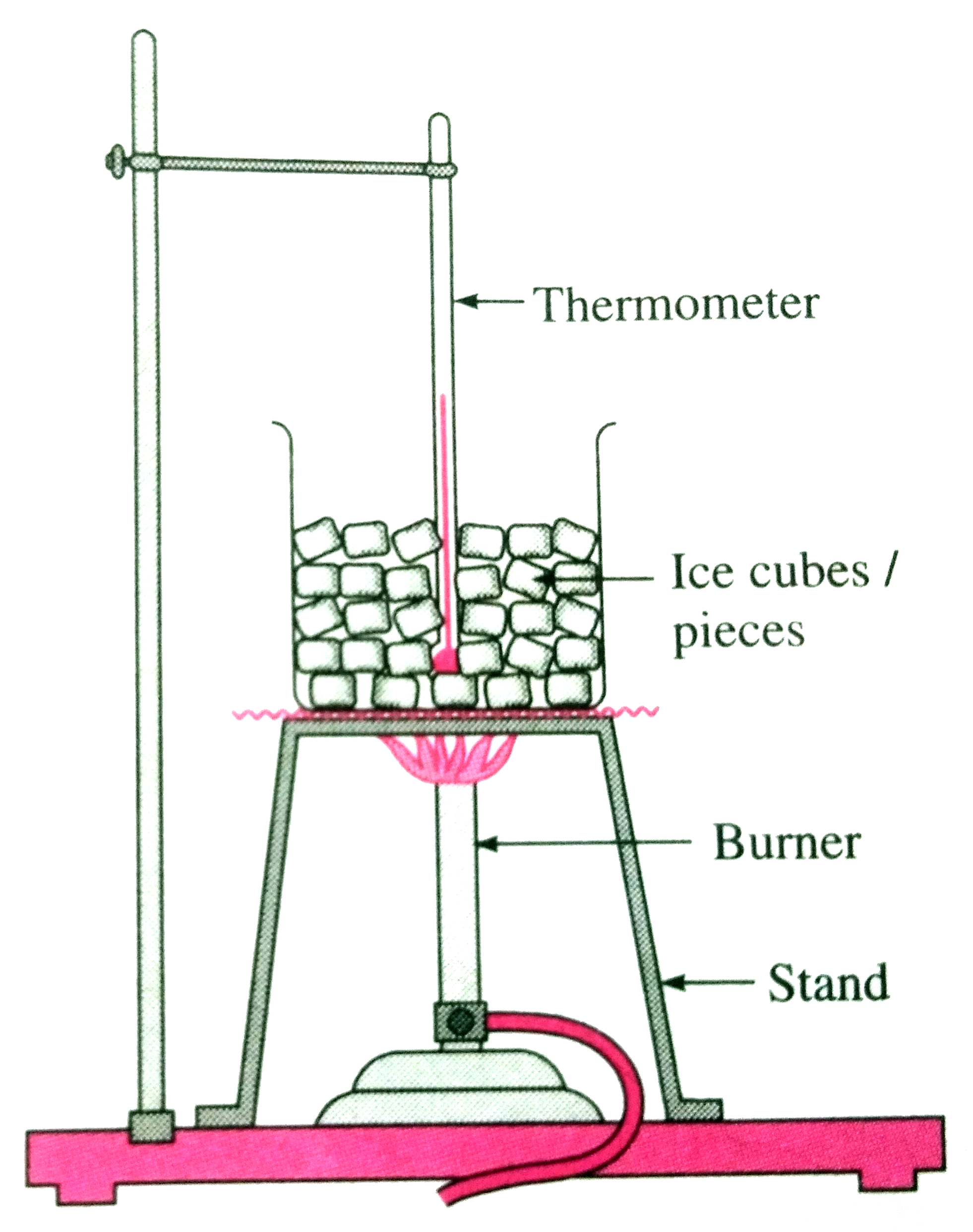
(2) Insert the bulb of a thermometer in ice and measure its temperature.
(3) Put the beaker on a stand and heat the ice using a burner.
(4) Record the temperature using the thermometer after every minute.
(5) As the ice is heated,it starts melting. Stir the mixture of ice and water.
(6) Continue the heating even after ice starts melting.
(7) Draw a graph of temperature versus time.
Define latent heat of fusion
OR
What is latent heat of fusion ? State its units.
View Solution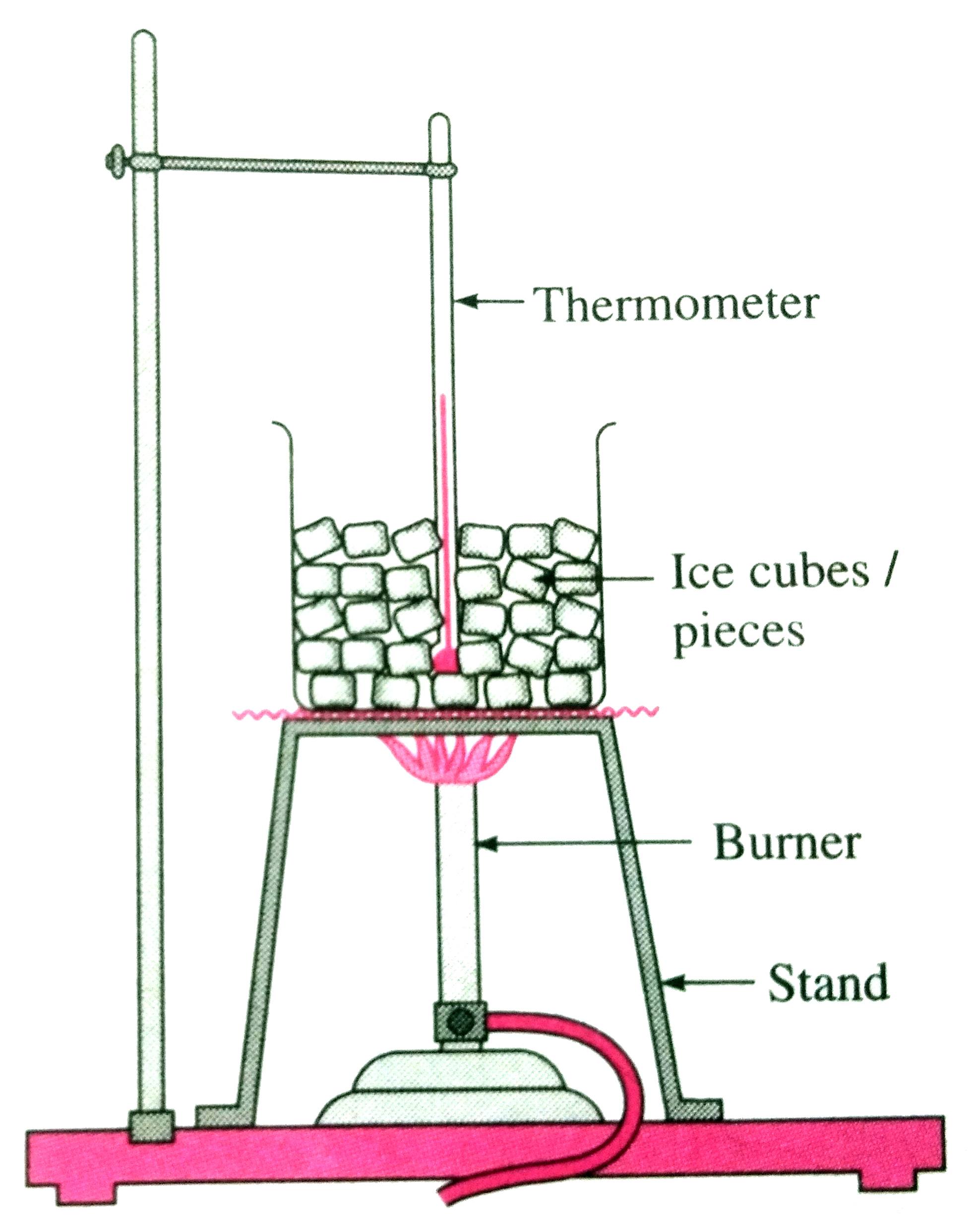
(1) Take a few pieces of ice in a glass beaker as shown in figure.
(2) Insert the bulb of a thermometer in ice and measure its temperature.
(3) Put the beaker on a stand and heat the ice using a burner.
(4) Record the temperature using the thermometer after every minute.
(5) As the ice is heated,it starts melting. Stir the mixture of ice and water.
(6) Continue the heating even after ice starts melting.
(7) Draw a graph of temperature versus time.
Define specific latent heat of fursion. OR
What is specific latent heat of fusion? State its units.
View Solution(1) Take a few pieces of ice in a glass beaker as shown in figure.
(2) Insert the bulb of a thermometer in ice and measure its temperature.
(3) Put the beaker on a stand and heat the ice using a burner.
(4) Record the temperature using the thermometer after every minute.
(5) As the ice is heated,it starts melting. Stir the mixture of ice and water.
(6) Continue the heating even after ice starts melting.
(7) Draw a graph of temperature versus time.
Explain the term latent heat of vaporization.
View Solution(1) Take a few pieces of ice in a glass beaker as shown in figure. (2) Insert the bulb of a thermometer in ice and measure its temperature. (3) Put the beaker on a stand and heat the ice using a burner. (4) Record the temperature using the thermometer after every minute. (5) As the ice is heated,it starts melting. Stir the mixture of ice and water. (6) Continue the heating even after ice starts melting. (7) Draw a graph of temperature versus time. Define boiling point of a liquid. OR What is boiling point of a liquid ?
View Solution(1) Take a few pieces of ice in a glass beaker as shown in figure. (2) Insert the bulb of a thermometer in ice and measure its temperature. (3) Put the beaker on a stand and heat the ice using a burner. (4) Record the temperature using the thermometer after every minute. (5) As the ice is heated,it starts melting. Stir the mixture of ice and water. (6) Continue the heating even after ice starts melting. (7) Draw a graph of temperature versus time. Define specific latent heat of vaporization. OR What is specific latent heat of vaporization?
View Solution(1) Take a few pieces of ice in a glass beaker as shown in figure. (2) Insert the bulb of a thermometer in ice and measure its temperature. (3) Put the beaker on a stand and heat the ice using a burner. (4) Record the temperature using the thermometer after every minute. (5) As the ice is heated,it starts melting. Stir the mixture of ice and water. (6) Continue the heating even after ice starts melting. (7) Draw a graph of temperature versus time. The specific latent heat of fusion of ice is 80 cal // g .Explain this statement .
View Solution(1) Take a few pieces of ice in a glass beaker as shown in figure. (2) Insert the bulb of a thermometer in ice and measure its temperature. (3) Put the beaker on a stand and heat the ice using a burner. (4) Record the temperature using the thermometer after every minute. (5) As the ice is heated,it starts melting. Stir the mixture of ice and water. (6) Continue the heating even after ice starts melting. (7) Draw a graph of temperature versus time. The specific latent heat of fusion of silver is 88.2kJ //kg . Explain this statement.
View Solution

