In following figs. Variation of volume by change of pressure is shown in Fig. A gas is taken along the path ABCDA. The change in internal energy of the tgas will be:
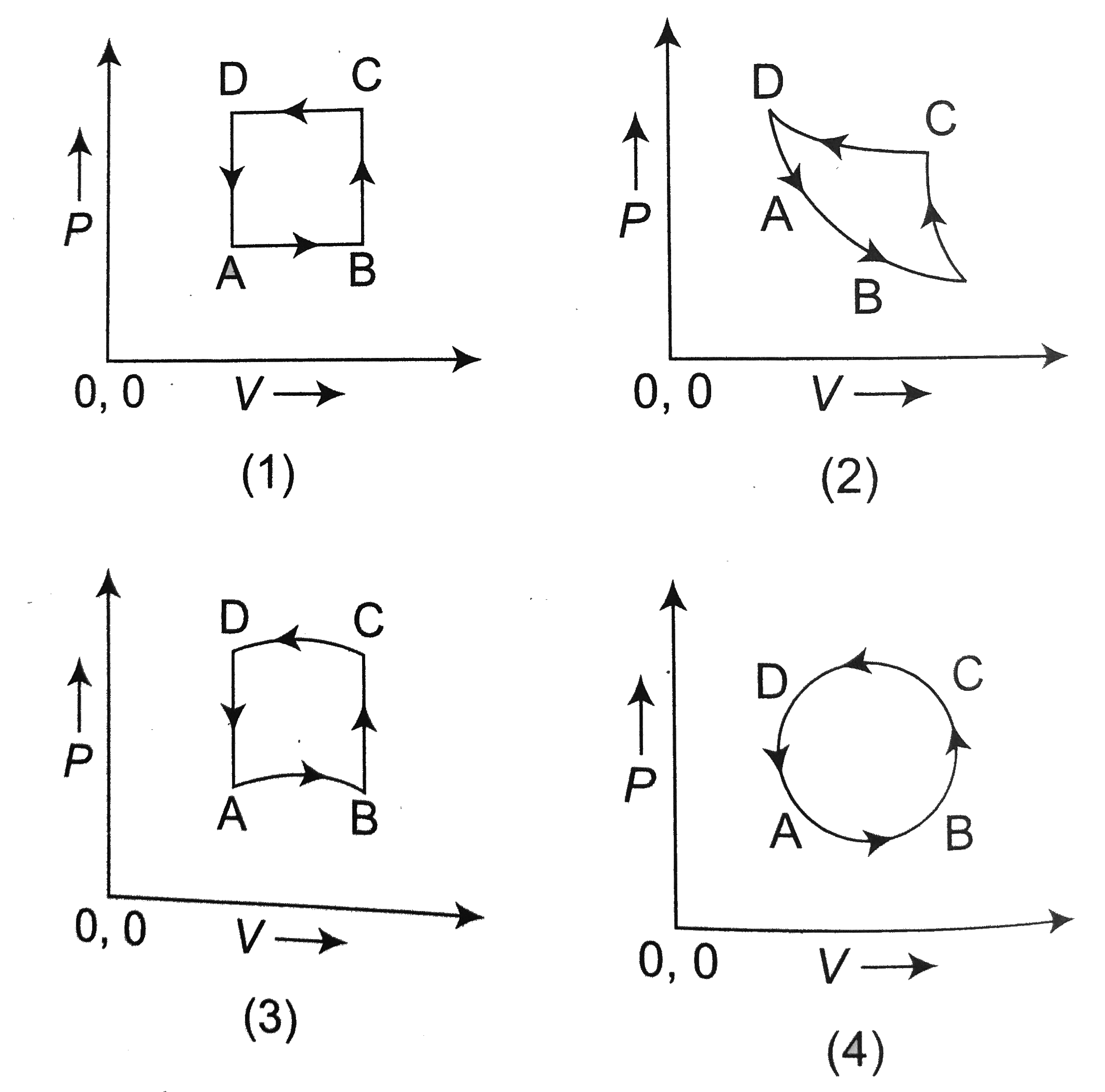
positive in all cases from (1) to (4)
positive in cases (1), (2) and (3) but zero in case (4)
negative in cases (1), (2) and (3) but zero in case (4)
zero in all the four cases.
The correct Answer is:D
Step by step video, text & image solution for In following figs. Variation of volume by change of pressure is shown in Fig. A gas is taken along the path ABCDA. The change in internal energy of the tgas will be: by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
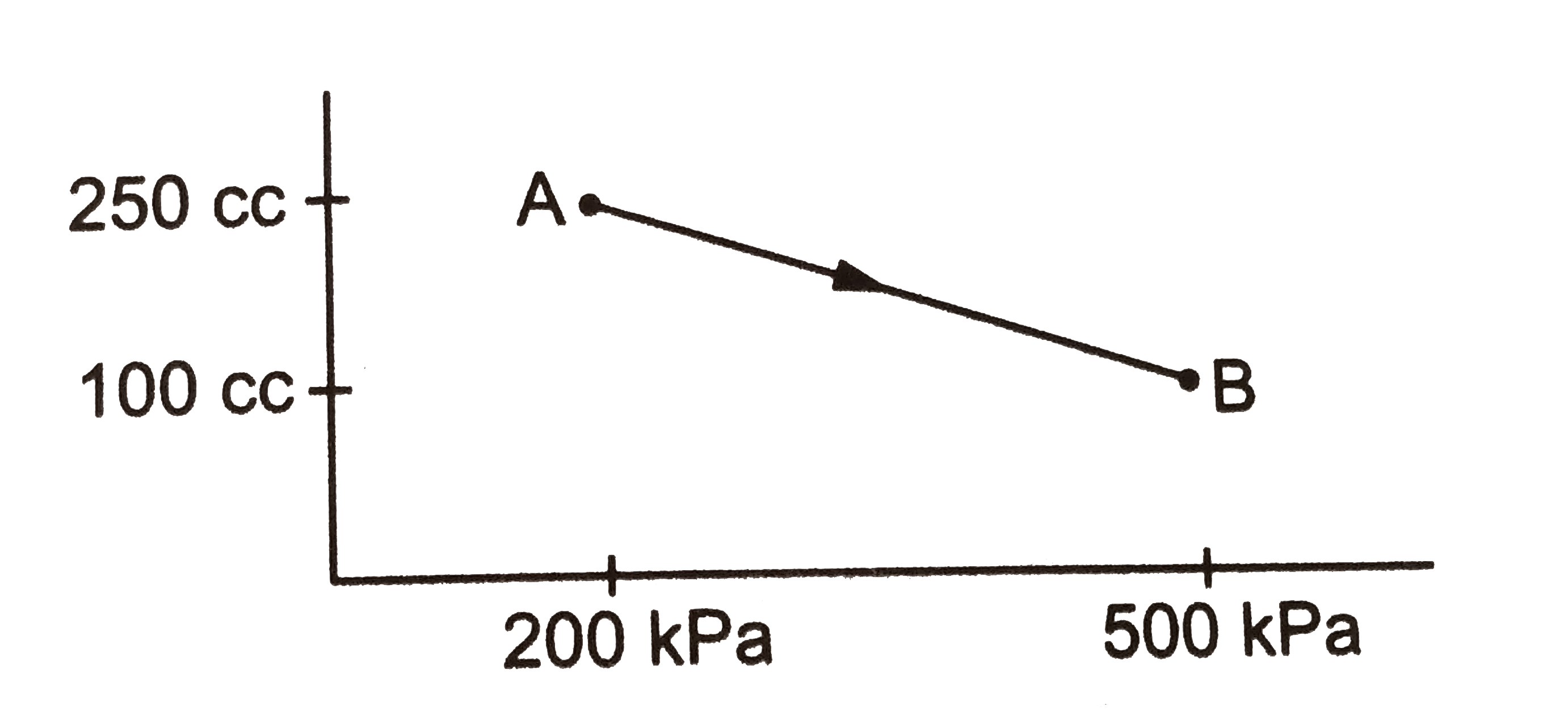
A gas is taken along the path AB as shown in fig, if 70 cal of heat is extracted from the gas in the process, calculate the change in the internal energy of the system.
View SolutionIn following figs. Variation of volume by change of pressure is shown in Fig. A gas is taken along the path ABCDA . The change in internal energy of the gas will be:
View SolutionA sample of an ideal gas is taken through the cyclic process abca and shown in the figure. The change in the internal energy of the gas along the path ca is -180J . The gas absorbs 250J of heat along the path ab and 60 J along the path bc. the work done by the gas along the path abc is:
View Solutionचित्र (i) से (iv) में दाब में परिवर्तन के साथ आयतन परिवर्तनदर्शाया गया है। एक गैस को पथ ABCDA के अनुदिश ले जाया जाता है। गैस की आन्तरिक ऊर्जा में परिवर्तन है :
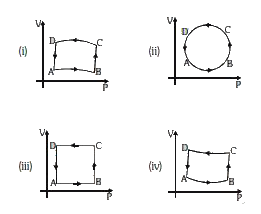
View SolutionChoose the correct option: In following figs. Variation of volume by change of pressure is shown in Fig. A gas is taken along the path ABCDA . The change in internal energy of the the gas will be:
View SolutionTwo moles of helium gas are taken along the path ABCD (as shown in Fig.) The work done by the gas is

View SolutionIn following figs. Variation of volume by change of pressure is shown in Fig. A gas is taken along the path ABCDA. The change in internal energy of the tgas will be:

View SolutionA mono-atomic gas is taken along path AB as shown. Calculate change in internal energy of the gas.
View Solution

