What is the structure ox XeF6?
A
Tetrahedral
B
Distorted octahedral
C
Octahedral
D
None of these
Video Solution
Text Solution
(b) For XeF6 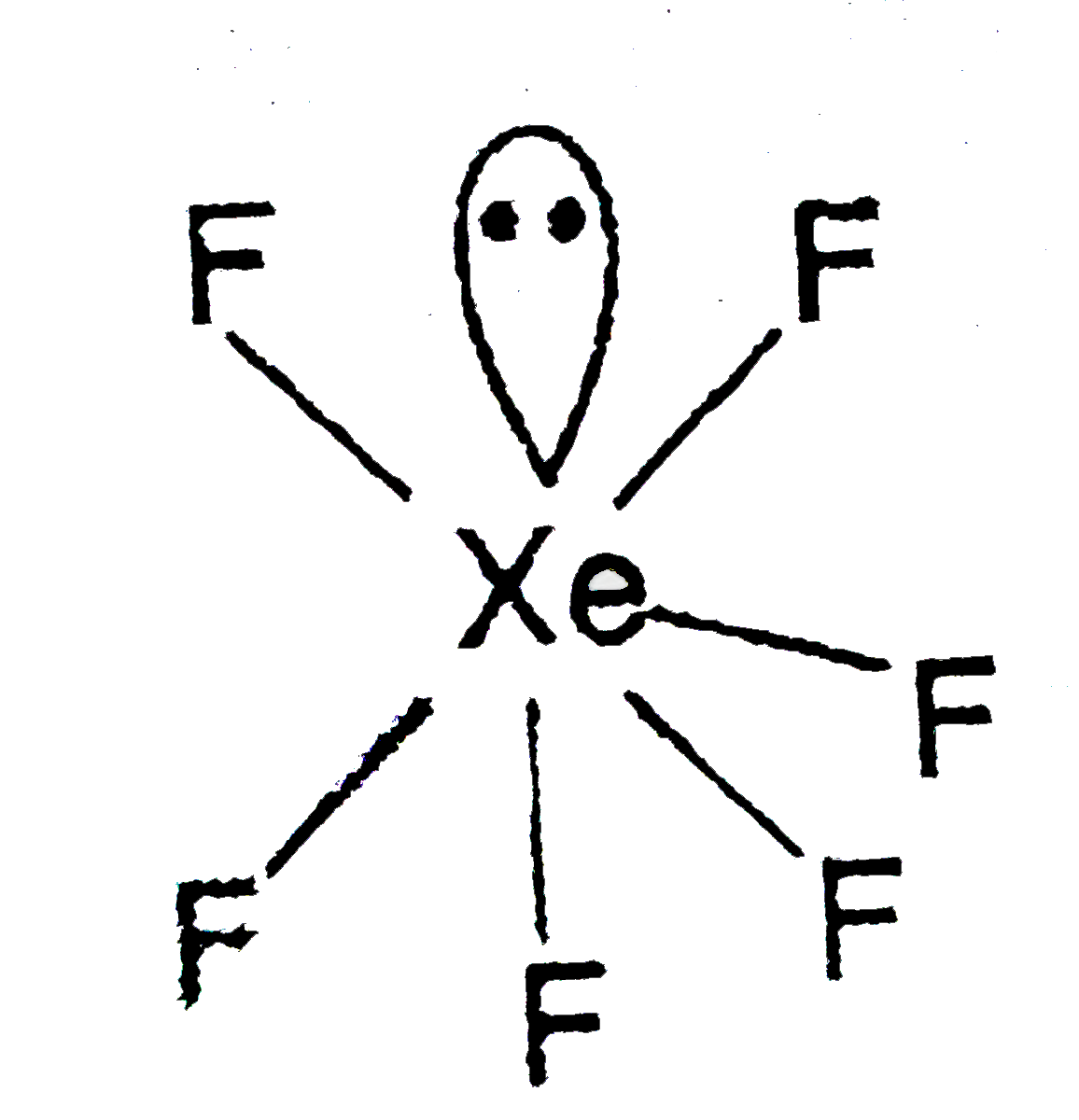
H=12[v+y−c+A]
=12[8+6−0+0]=7
⇒Hybridisation=sp3d3
Due to the presence of one lone pair of electron,XeF6 has distorted octahedral geometry.
|
Similar Questions
Knowledge Check
The structure of XeF6 is
Adistorted octahedralBtrigonal pyramidalCtetrahedralDnone of the aboveThe structure of XeF6 is
Adistorted octahedralBpyramidalCtetrahedralDnone of theseThe structure of XeF4 is
ATetrahedralBSquare planarCLinearDOctahedral.
