Assertion : Phenols are more acidic than aliphatic alcohols.
Reason : Phenoxides are stabilized by resonance.
Both A and R are correct and R is correct explanation of A
Both A and R are correct but R is not the correct explanation of A
A is correct but R is in correct
A is incorrect but R is correct
The correct Answer is:A
Step by step video, text & image solution for Assertion : Phenols are more acidic than aliphatic alcohols. Reason : Phenoxides are stabilized by resonance. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- ALCOHOLS, ETHERS, PHENOLBook:NARAYNAChapter:ALCOHOLS, ETHERS, PHENOLExercise:Level-I (H.W)
Explore 9 Videos
- ALCOHOLS, ETHERS, PHENOLBook:NARAYNAChapter:ALCOHOLS, ETHERS, PHENOLExercise:Level-II (H.W)
Explore 8 Videos
- ALCOHOLS, ETHERS, PHENOLBook:NARAYNAChapter:ALCOHOLS, ETHERS, PHENOLExercise:Level-III (Properties of phenol)
Explore 6 Videos
- 18TH GROUP ELEMENTSBook:NARAYNAChapter:18TH GROUP ELEMENTSExercise:LEVEL - VI
Explore 34 Videos
- ALDEHYDES, KETONES & CARBOXYLIC ACIDSBook:NARAYNAChapter:ALDEHYDES, KETONES & CARBOXYLIC ACIDSExercise:CHECK YOUR GRASP
Explore 6 Videos
Similar Questions
Knowledge Check
Phenols are more acidic than aliphatic alcohols because
Aphenoxide ion is stabilised by resonanceBphenols are more soluble in polar solventsCphenoxide ion do not have resonanceDalcohols do not loose H-atom at allPhenol is more acidic than :
AC2H5OHBCH3COOHC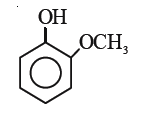 D
D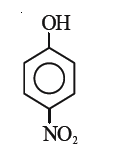
Phenol is more acidic than
A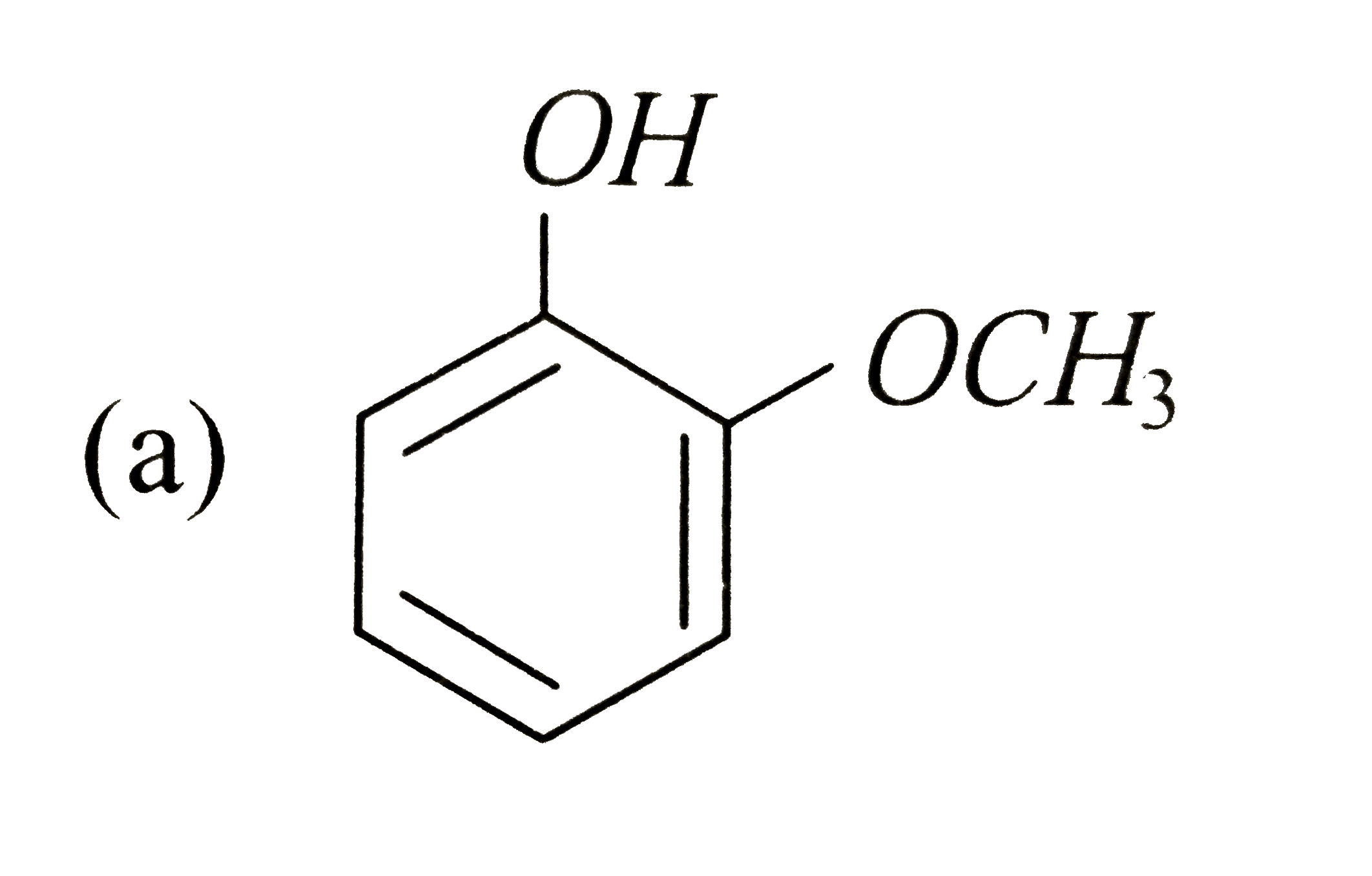 B
B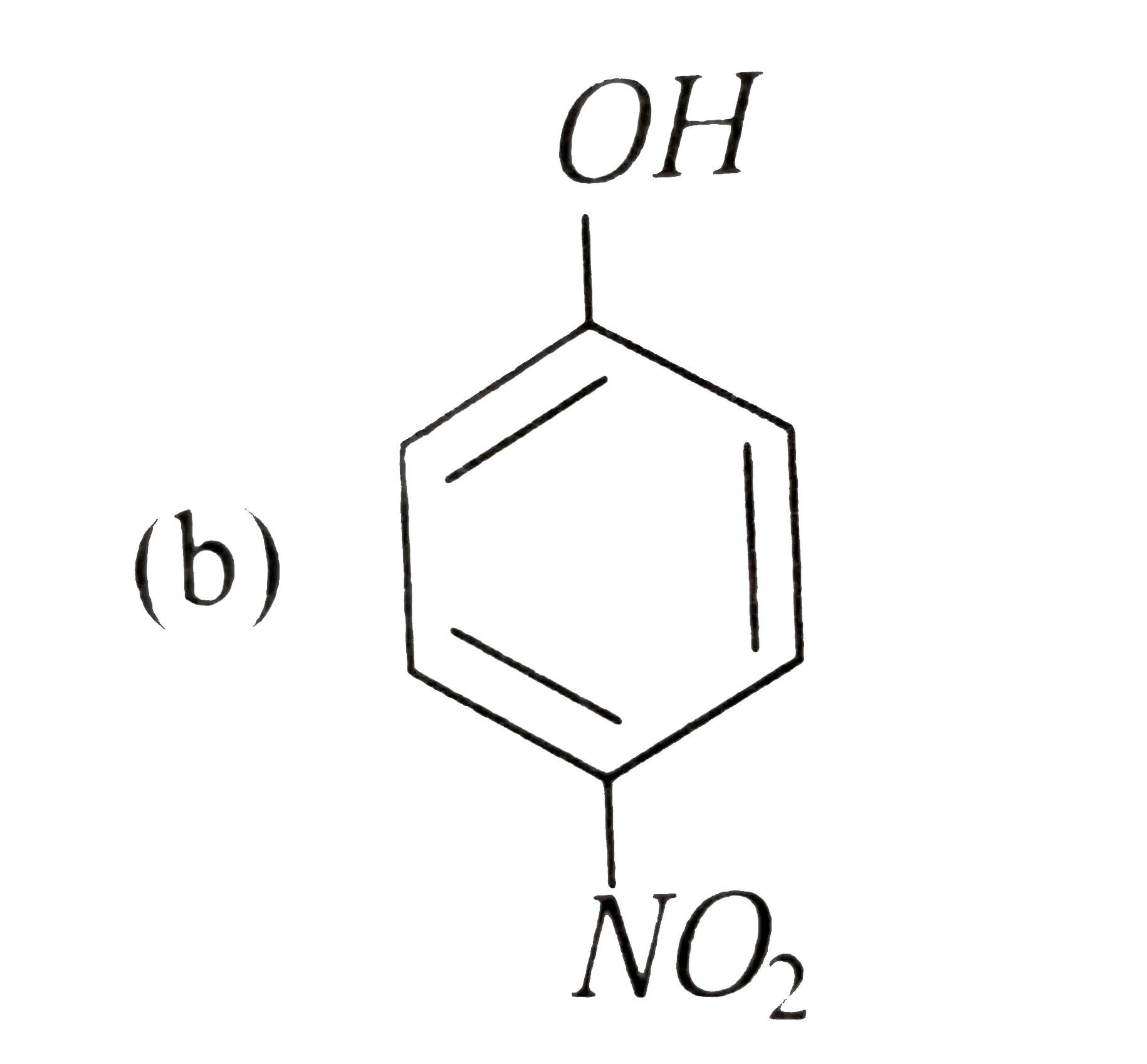 CC2H2DBoth (a) and (c)
CC2H2DBoth (a) and (c)
Assertion : Phenol is more acidic than ethanol.
Reason : Phenoxide ion is resonance stabilized.
View SolutionPhenols are more acidic than alcohols because
View SolutionPhenols are more acidic than alcohols because
View SolutionAssertion (A) : Phenol is strongly acidic than ethanol.
Reason (R) : Phenoxide ion is more stabilized by resonance than ethoxide ion.
View SolutionPhenol are more acidic than alcohols because
View Solution
