Phenols are much more acidic than alcohols. Explain.
Step by step video & image solution for Phenols are much more acidic than alcohols. Explain. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
How would you account for the following :
(i) Phenols are much acidic than alcohols.
(ii) The boiling points of ethers are much lower than those of alcohols of comparable molecular measses.
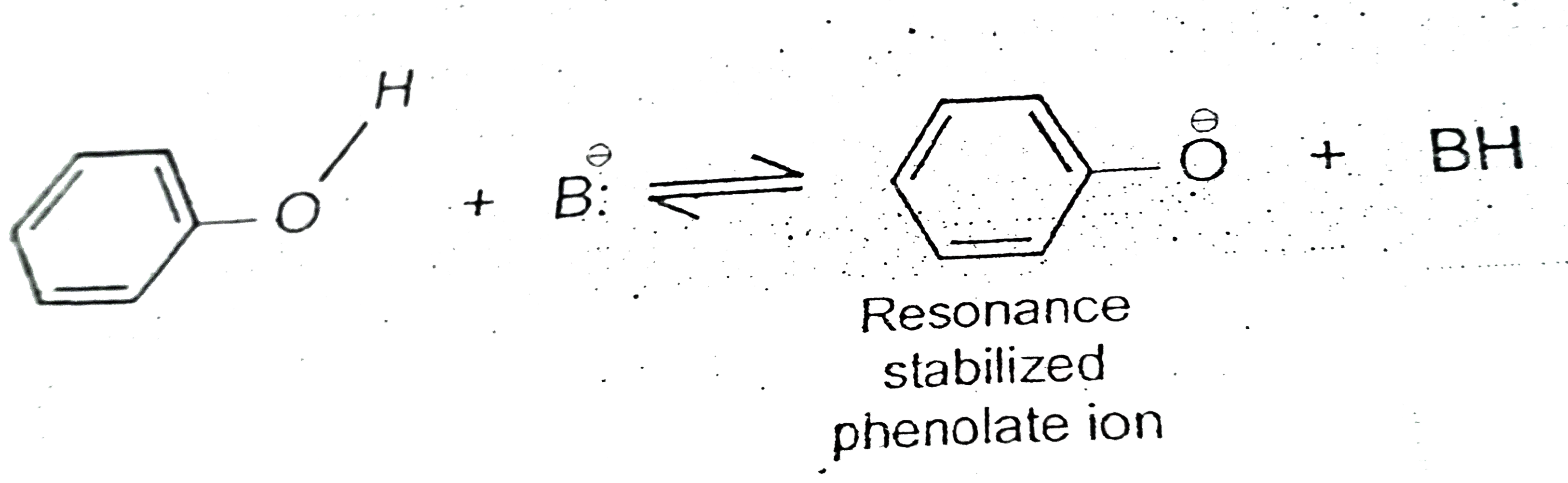
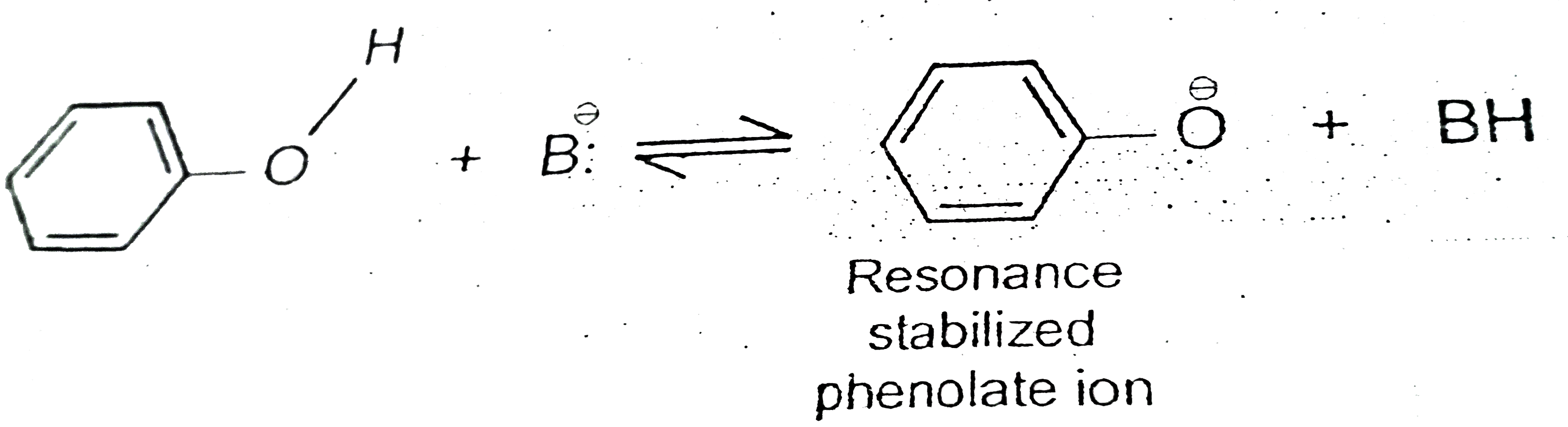
View SolutionPhenols are more acidic than aliphatic alcohols acidity of phenols can be further increased by the introduction of electron withdrawing groups in aromatic ring .Acidic nature of phenol is because of the resonance stabilization of phenoxide ion
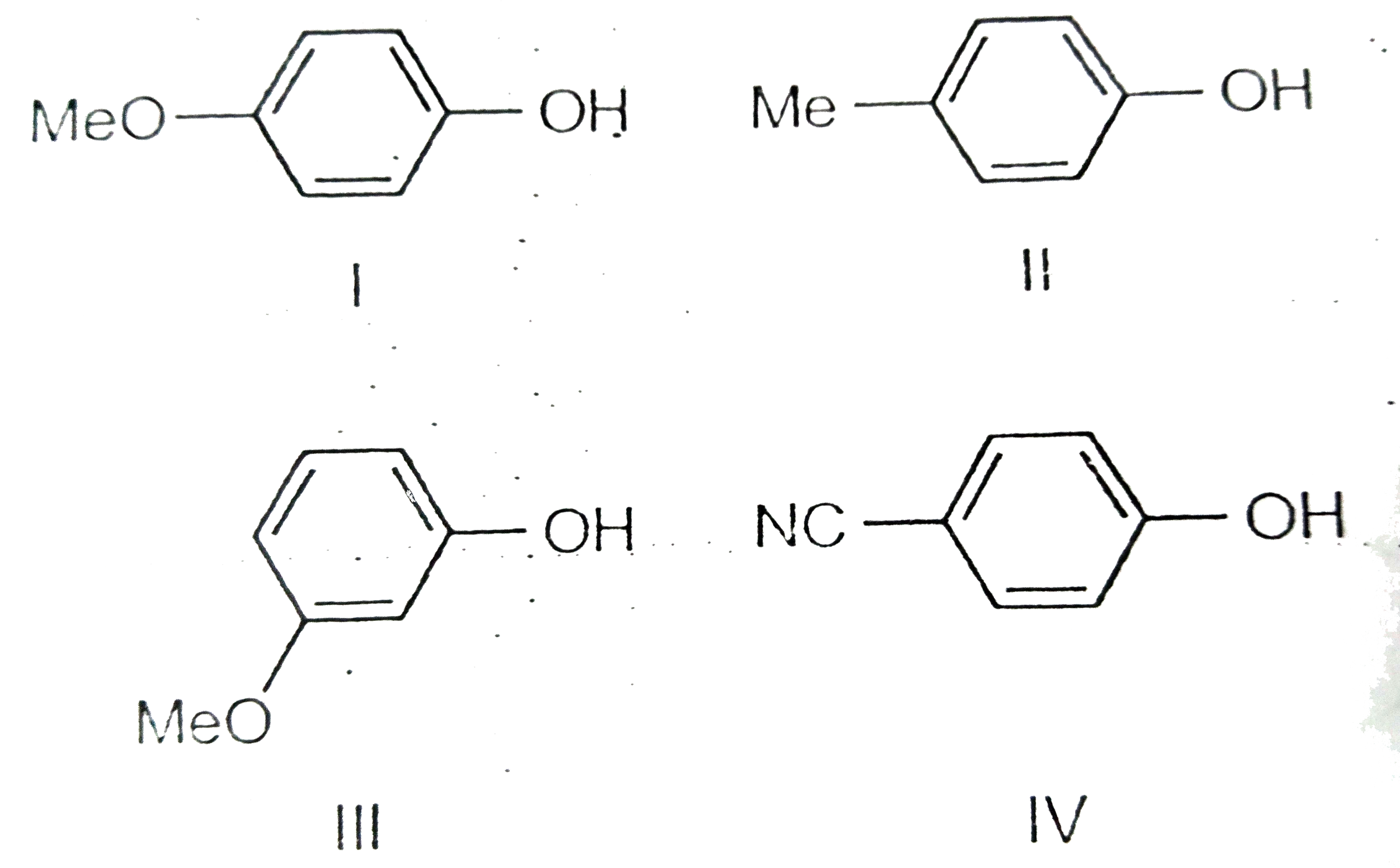
Arrange the given phanols in the increasing order of acidic strength
View Solution
Knowledge Check
Phenols are more acidic than alcohols because
Aphenols are more soluble in water than alcohols are.Bthe phenoxide ion is stabilized by resona-nce but resonance is not possible in the alkoxide ion.Cresonance stabilization in the phenoxide ion is greater than that in the alkoxide ion.Dphenol are stabilized by resonance but alcohol are not.Phenols are more acidic than alcohols because
Aphenoxide ion is stabilized by resonanceBphenols are more soluble in polar solventsCphenoxide ions do not exhibit resonanceDalcohols do not lose H atoms at allPhenol are more acidic than alcohols because
Aphenoxide ion is stabilised by resonanceBphenols are more soluble in polar solventsCphenols ions do not exhibit resonanceDalcohols donot lose H atoms at all.
Phenols are more acidic than aliphatic alcohols acidity of phenols can be further increased by the introduction of electron withdrawing groups in aromatic ring .Acidic nature of phenol is because of the resonance stabilization of phenoxide ion
Which of the following will evolve CO2 gas with aqueous NaHCO3?
View SolutionPhenol is more acidic than alcohol because :
View SolutionPhenols are more acidic than alcohols and CH3NO2 because
View SolutionAssertion : Phenols are more acidic than aliphatic alcohols.
Reason : Phenoxides are stabilized by resonance.
View SolutionPhenols are more acidic than aliphatic alcohols because
View Solution

