The monomer that undergo radical polymerisation most easily is :
A
CH2=CH2
B
C6H5CH=CH2
C
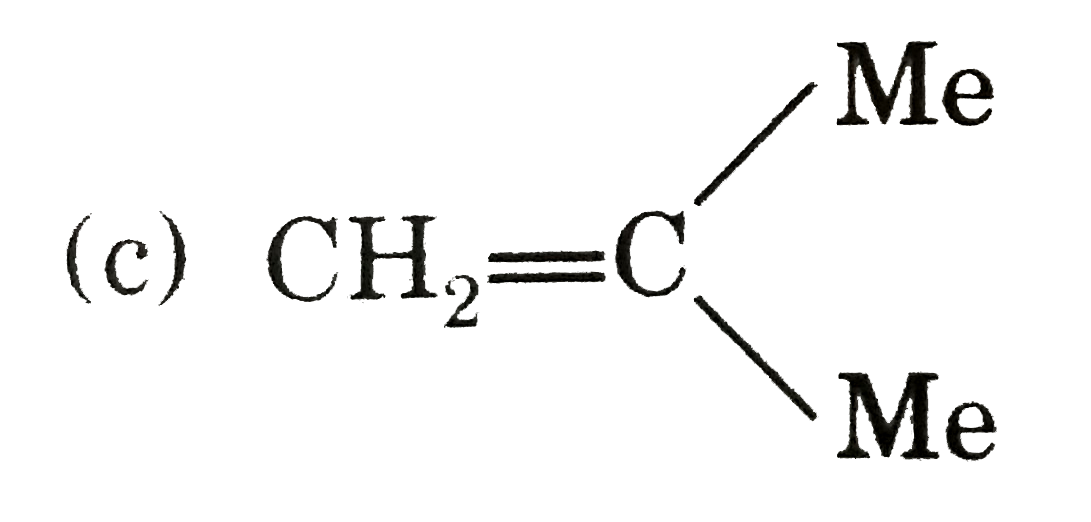
D
CH3−CH=CH2
Text Solution
The correct Answer is:b
|
Similar Questions
Knowledge Check
Assertion (A) Olefinic monomers undergo addition polymerisation
Reason (R ) Polymerisation of vinyl chloride is initiated by peroixdes/persulphatesAAssertion and reason both are correct statements and reason does not explain assertion.BAssertion and reason both are correct statements but reason explains the assertion.CBoth assertion and reason are wrong statements.DAssertion is correct statement and reason is wrong statement.Assertion (A) Olefinic monomers undergo addition polymerisation
Reason (R ) Polymerisation of vinyl chloride is initiated by peroixdes/persulphatesAAssetion and Reason both are correct statement But reason does not explain AssertionBAssertion and Reason both are correct statements and Reason explains the Assertion.CBoth assertion and reason are wrong sttement sDAssertion is correct statement and Reason is worng statement.Which of the following monomers can unergoes radical, cationic as well as anionic polymerisation with equal ease?
ACH3−C∣CH3≡CH2BC6H5−CH=CH2CCH2=CH−CNDCH2=CH2
Explain the free radical polymerisation of styrene.
View SolutionWhy should one always use purest monomer in free radical polymerisation reaction ?
View SolutionPolymer obtained by polymerisation of monomers
View SolutionWe should use purest monomer in free radical polymerisation. True/False.
View SolutionWhat is mode of free radical polymerisation in alkenes ?
View Solution

