Perovaskite, a mineral containing calcium, oxygen & titanium crystallizes in the given unit cell
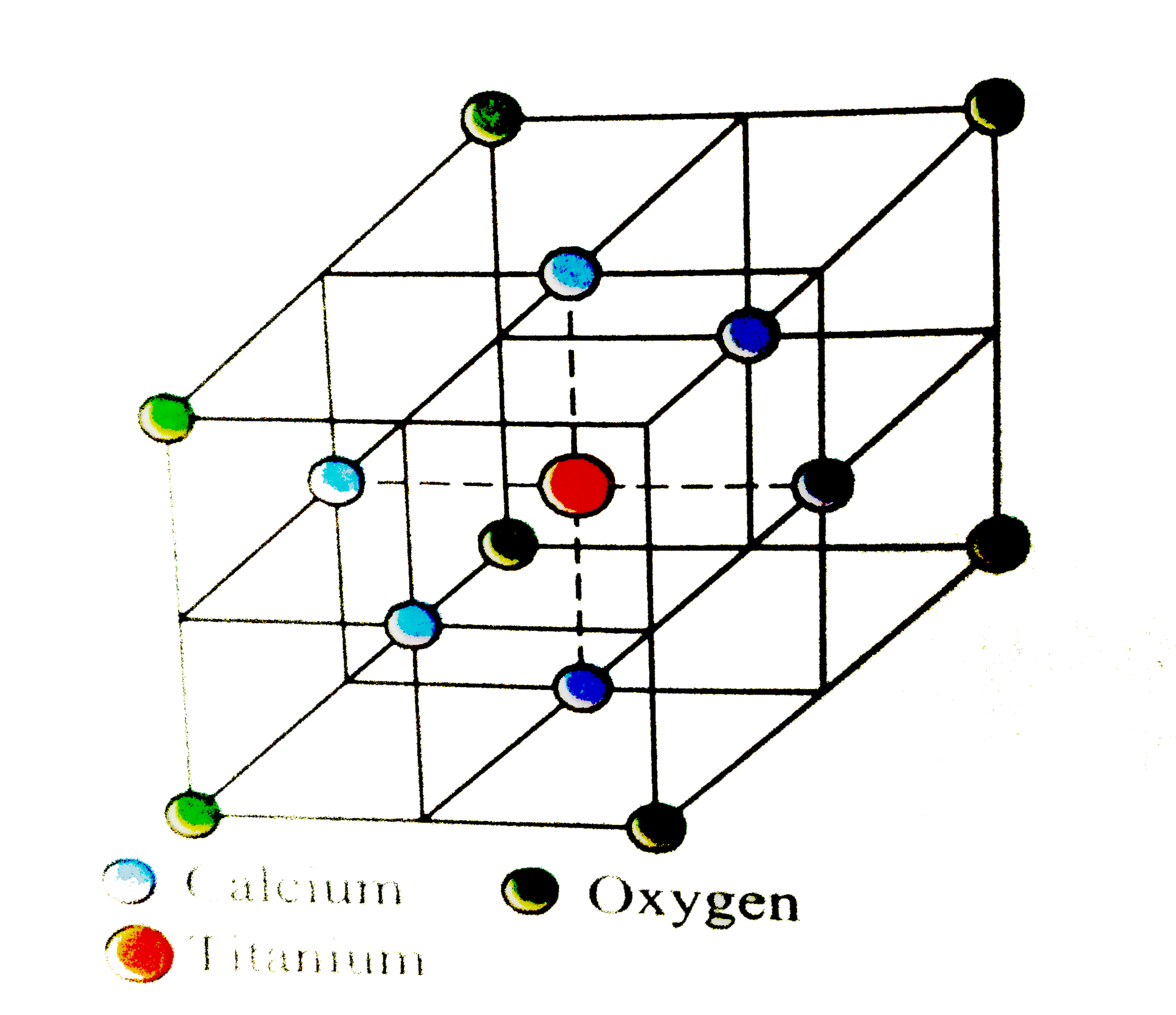
Total number of atom present in a unit cell is
A
3
B
4
C
5
D
6
Video Solution
Text Solution
No. of Ca atom =8×18=1
No. of O-atom=6×12=3
No. of Ti-atom=1xx1=1: Total no. of atom=5
|
Similar Questions
Knowledge Check
Perovaskite, a mineral containing calcium, oxygen & titanium crystallizes in the given unit cell
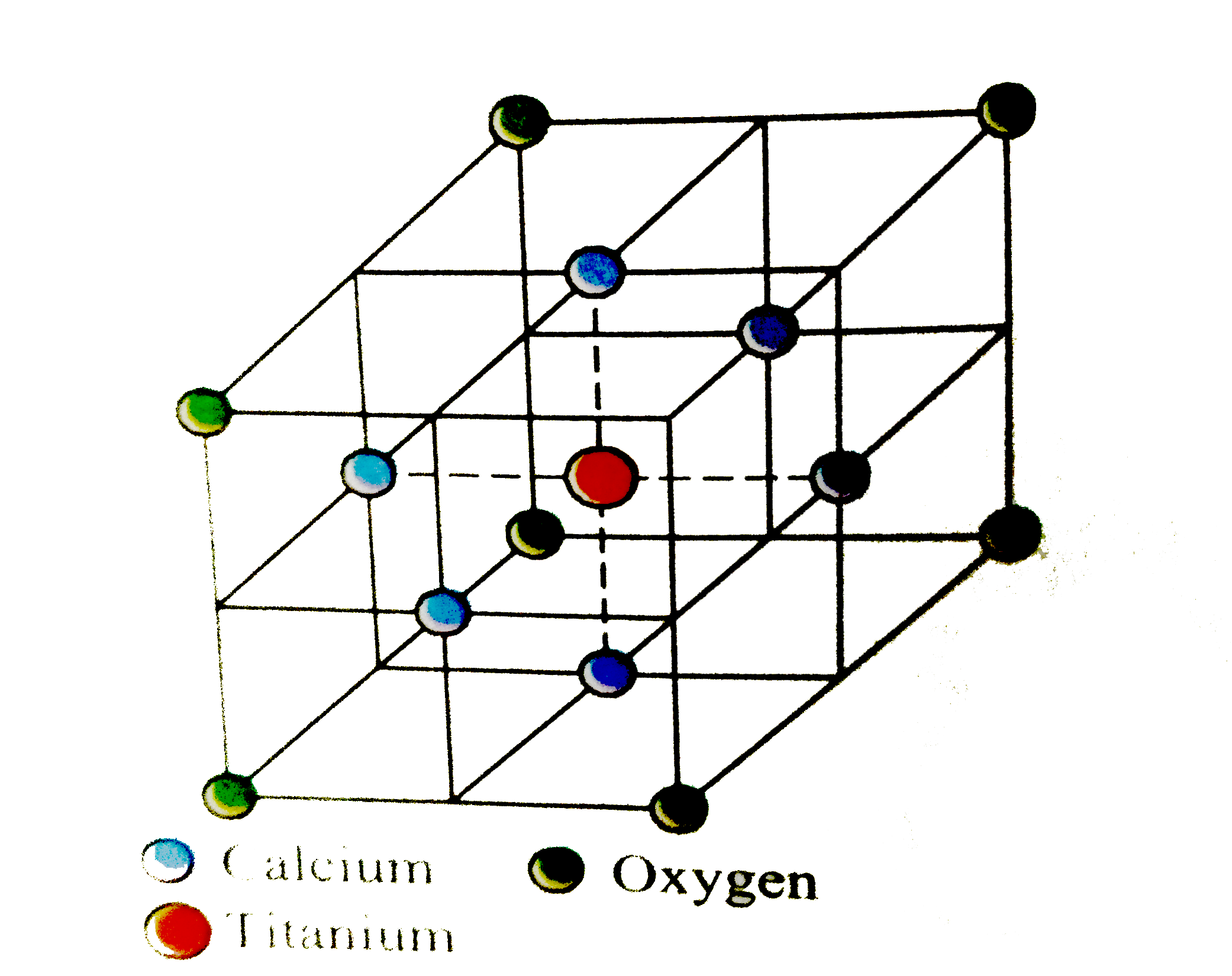
The oxidation number of titanium in the perovskite isA+5B+4C+3D+2Perovaskite, a mineral containing calcium, oxygen & titanium crystallizes in the given unit cell
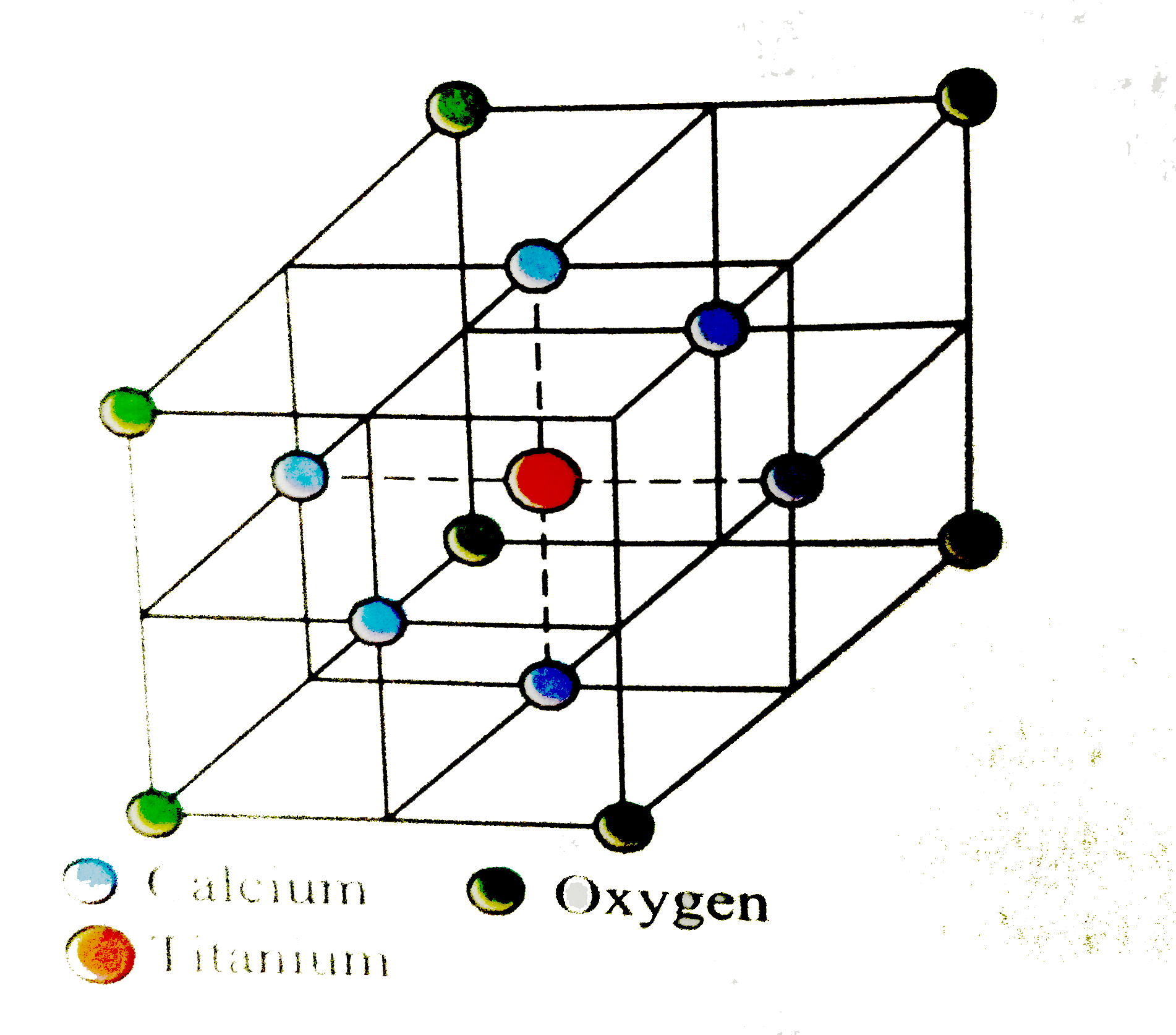
If oxygen atoms are removed from alternate position then the formula of perovskite isACaTiOBCaTi2OCCaTiO2DCa3Ti2ONumber of atoms in fcc unit cell is
A1B2C3D4
How many number of atoms are present in fcc unit cell?
View SolutionIn a body centred unit cell, the number of atoms present is
View SolutionIn a face centered unit cell (fcc) the number of atoms present
View SolutionIf the total number of atoms per unit cell is 2, the unit cell is
View SolutionThe number of atoms present in a simple cubic unit cell are-
View Solution
