Perovaskite, a mineral containing calcium, oxygen & titanium crystallizes in the given unit cell
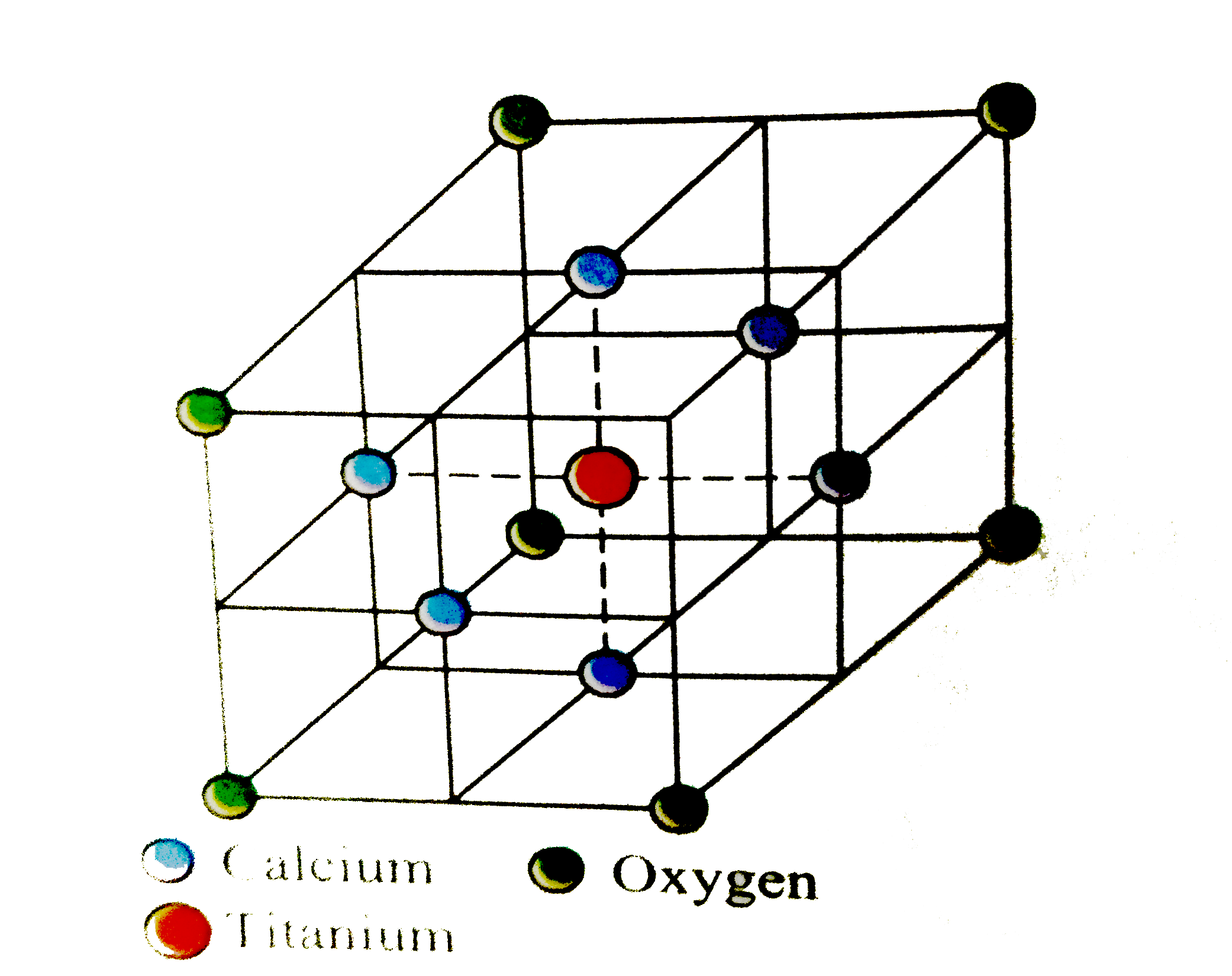
The oxidation number of titanium in the perovskite is
+5
+4
+3
+2
CaTiO3,2+x−6=0,x=4
Similar Questions
Perovskite is mineral containing calcium, oxygen and titanium, in which oxygen atoms are at the face centres, calcium atoms are at the corners and titanium atoms at the centre of the cube. Oxidation number of titanium in the mineral is
View SolutionWhat is the percent by mass of titanium in rutile, a mineral that contain Titanium and oxygen, if structure can be described as a closet packed array of oxide ions, with titanium in one half of the octahedral holes. What is the oxidation number of titanium ?
View SolutionWhat is the percent by mass of titanium in rutile, a mineral that contain Titanium and oxygen, if structure can be described as a closet packed array of oxide ions, with titanium in one half of the octahedral holes. What is the oxidation number of titanium ?
View SolutionPerovskite is a mineral composed of Ca, Ti and oxygen, cations of titanium lie at the centre, oxides ions at the face centres and calcium ions lie at corners. In this compound the oxidation number of Titanium is +x . Find the value of x?
View SolutionA mineral of titanium (perovskite) is found to contain calcium ions at the corners, oxygen atoms at the face centres and titanium atoms at the centre of the cube. The oxidation state of titanium in the mineral is
View SolutionThe mineral rutile is an oxide of titanium containing 39.35% oxygen and is isomorphous with cassiterite (SnO2). The atomic weight of titanium is
View SolutionThe figure below shows a unit cell of the mineral Perovskite ( the titanium atom is at the centre of the cube ). Which of the following is a correct chemical formula for this mineral ?

View SolutionPerovskite is a mineral composed of Ca,Ti and oxygen, cations of titanium lie at the centre, oxides ions at the face centres and calcium ions lie at corners. In this compound the oxidation number of Titanium is +x. Find the value of x?
View Solution
