R−CH2−O||C−OHP/Cl2−−−→H2OR−Cl∣CH−O||C−OH
Which of the following are intermediate species reponsible for product ?
A
R−CH2−O||C−Cl
B
R−.CH−O||C−OH
C
R−CH=C∣Cl−OH
D
R−C∣ClH−O||C−Cl
Text Solution
The correct Answer is:A, C, D
|
Topper's Solved these Questions
- CABOXYLIC ACIDS AND ITS DERIVATIVESBook:HIMANSHU PANDEYChapter:CABOXYLIC ACIDS AND ITS DERIVATIVESExercise:More Than One Correct (Q.26 To Q.50)
Explore 25 Videos
- CABOXYLIC ACIDS AND ITS DERIVATIVESBook:HIMANSHU PANDEYChapter:CABOXYLIC ACIDS AND ITS DERIVATIVESExercise:More Than One Correct (Q.50 To Q.50)
Explore 1 Video
- CABOXYLIC ACIDS AND ITS DERIVATIVESBook:HIMANSHU PANDEYChapter:CABOXYLIC ACIDS AND ITS DERIVATIVESExercise:Level 2 (Q.101 To Q.120)
Explore 20 Videos
- BIOMOLECULESBook:HIMANSHU PANDEYChapter:BIOMOLECULESExercise:Match The Column
Explore 6 Videos
- CARBONYL COMPOUNDSBook:HIMANSHU PANDEYChapter:CARBONYL COMPOUNDSExercise:Subjective Type Problems
Explore 10 Videos
Similar Questions
Knowledge Check
CH3−OH∣CH−O∣∣C−CH2−CH3 will respond to
Aonly Fehling solutionBOnly Tollen's reagentCBoth Tollen's reagent and Fehling solutionDnone of theseCompound having −O∣∣C−CH3 group reacts rapidly with halogen in presence of a base to form haloform . The reaction is known as haloform. R−O∣∣C−CH3X2+ӨOH−−−−→R−O∣∣C−ӨO+CHX3
Mechanism :
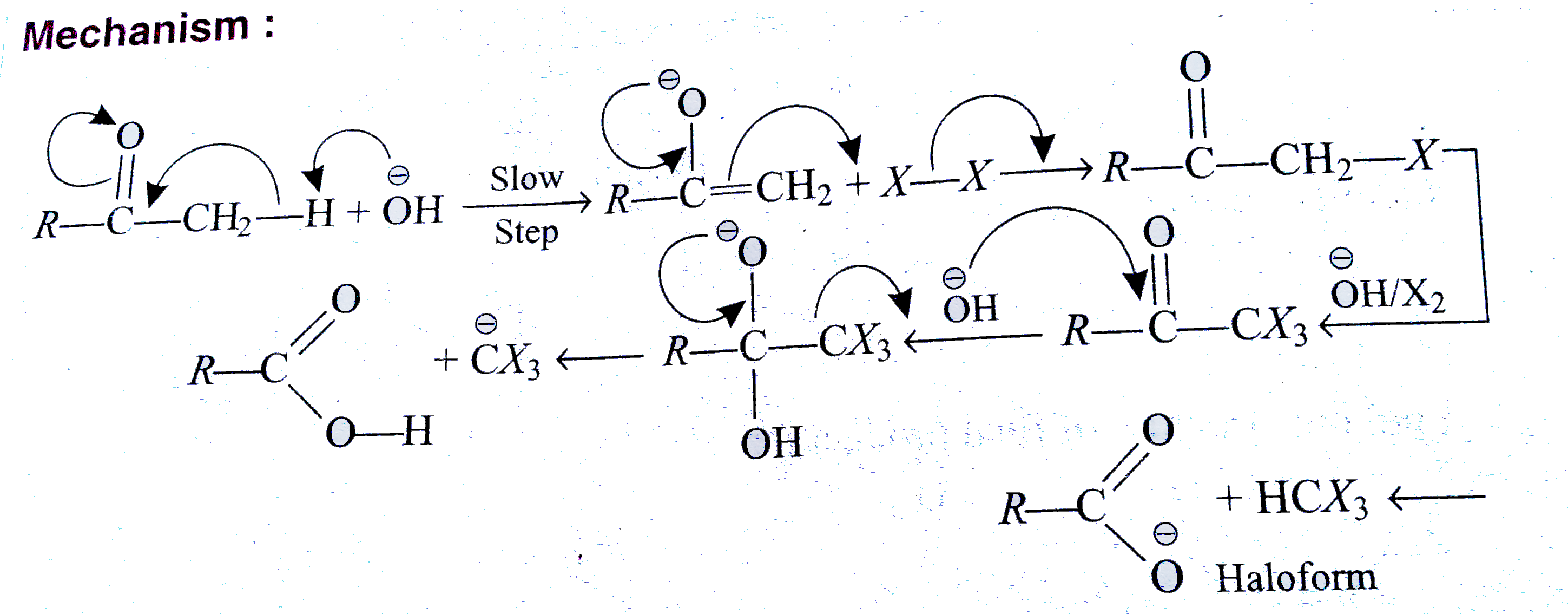
Consider the following reactions :
Reaction I →Ph−O∣∣C−CH3Cl2,ӨOH−−−−→Ph−O∣∣C−ӨO+CHCl3
Reaction II →Ph−O∣∣C−CD3Cl2ӨOH−−−→,Ph−O∣∣C−ӨO+CDCl3
Which of the following is correct comparison of the rate of reaction I and II ?ArI>rIIBrI<rIICrI=rIIDCannot be deteminedThe molecules CH3−O∣∣C−CH2−O∣∣C−O−C2H5 and CH3−OH∣C−OH−O∣∣C−O−C2H5 are
Ageometrical isomersBTautomersCDiastereomersDMetamers

