R−COOH+(CF3CO)2O→XR'−OH−−−−→Y+Z:
Y is R'−C||O−O−R
X is R−C||O−O−C||O−CF3
Y is R−C||O−O−R'
Z is CF3−COOH
The correct Answer is:B, C, D
Topper's Solved these Questions
- CABOXYLIC ACIDS AND ITS DERIVATIVESBook:HIMANSHU PANDEYChapter:CABOXYLIC ACIDS AND ITS DERIVATIVESExercise:More Than One Correct (Q.26 To Q.50)
Explore 25 Videos
- CABOXYLIC ACIDS AND ITS DERIVATIVESBook:HIMANSHU PANDEYChapter:CABOXYLIC ACIDS AND ITS DERIVATIVESExercise:More Than One Correct (Q.50 To Q.50)
Explore 1 Video
- CABOXYLIC ACIDS AND ITS DERIVATIVESBook:HIMANSHU PANDEYChapter:CABOXYLIC ACIDS AND ITS DERIVATIVESExercise:Level 2 (Q.101 To Q.120)
Explore 20 Videos
- BIOMOLECULESBook:HIMANSHU PANDEYChapter:BIOMOLECULESExercise:Match The Column
Explore 6 Videos
- CARBONYL COMPOUNDSBook:HIMANSHU PANDEYChapter:CARBONYL COMPOUNDSExercise:Subjective Type Problems
Explore 10 Videos
Similar Questions
The oxidation of which of the following compounds with KMnO4,ΘOH/Δ followed by treatement of acid will give benzoic acid ?
View SolutionConsider the following reaction sequence:
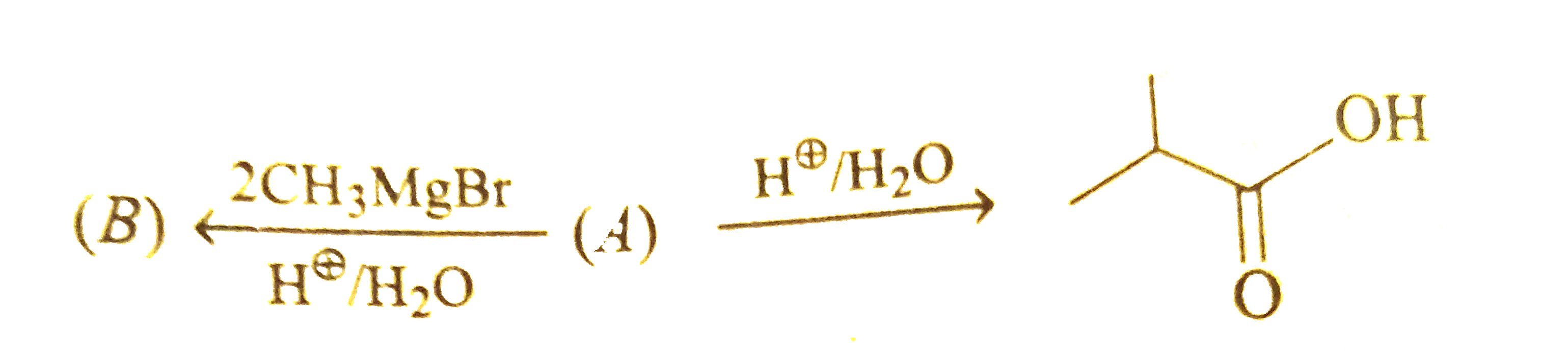
View SolutionThe major product of the reaction :
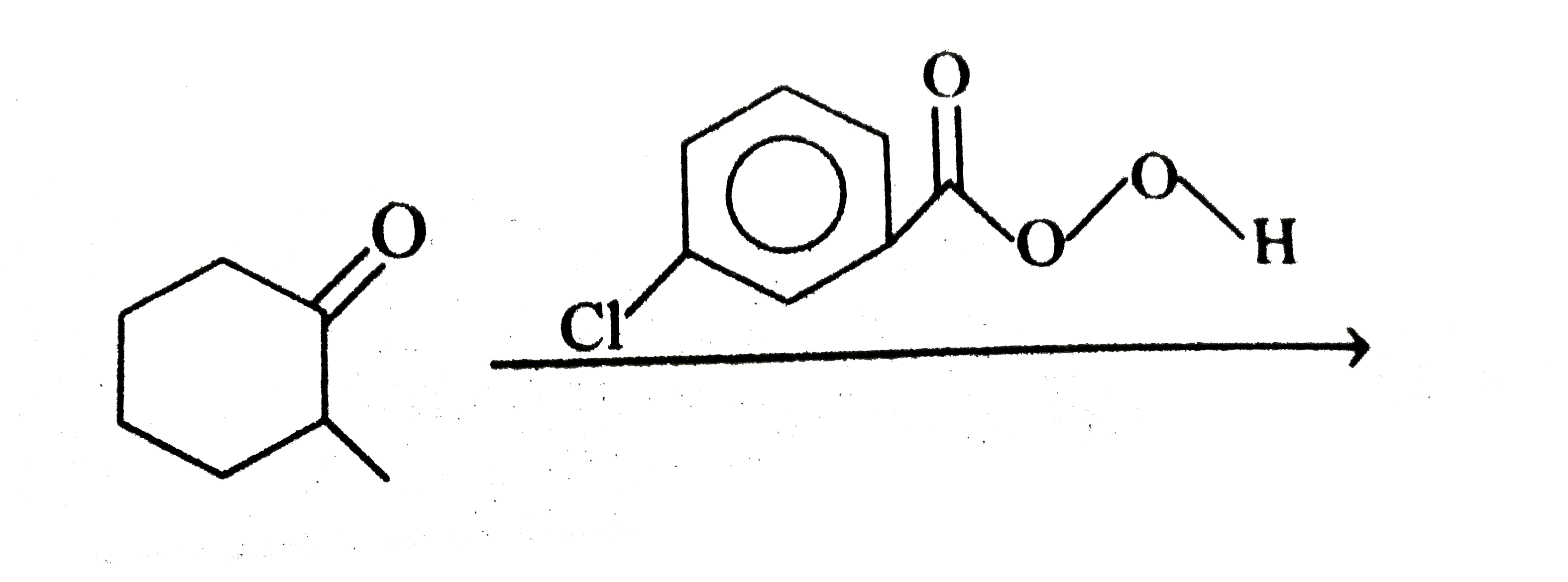
View Solution

View SolutionFind out final product of following reaction :

View SolutionR−O∣∣C−OH[?]−−→RCH2−OH
Here reagent is
View SolutionFind out A, B, C , D in following reaction (1) CH_(3) - CH = CH - CHO overset(A) to CH_(3) - CH = CH - COOH (2) CH_(3) - CH = CH - CHO overset(B) to CH_(3) - CH = CH - CH_(2) - OH (3) R - COOH overset(C) to R - CH_(2) - OH (4) R - COCl overset(D) to R - CHO
View SolutionR - CO - R'overset(HCN) to A overset(B) to R - underset(CH_(2)-NH_(2)) underset(|) overset(OH) overset(|) (C) - R A and B are
View SolutionFor the following reaction R - CN overset(H_(3)O^(+)) to R-COOH
View SolutionR−COOHA −−−−→R−COCl. What is A
View SolutionR-COOH to R-CH_(2)-OH . Name the reagent used in this conversion.
View SolutionConsider the gas-phase hydration of hexafluoroacetone, (CF_(3))_(2) CO : (CF_(3))_(2)CO(g) + H_(2)O (g) underset(k_(r))overset(k_(f))hArr(CF_(3))_(2)C(OH)_(2)(g) At 76° C, the forward and reverse rate constants are k_(f) = 0.13 M^(-1) s^(-1) and k_(r) = 6.02 x× 10^(-4) s^(-1) . What is the value of the equilibrium constant K_(c) ?
View SolutionThe reduction of R-COOH to R-CH_(2)OH can be effected by :
View SolutionWhat is the reagent A used in the following equation?
R−COOH−−→AR−CH2OH
View SolutionIn the reaction , CH3COOHCa(OH)2−−−−−→AHeat−−→BNH2OH−−−−→C, C, C is :
View Solution

