The intemediates formed during the reaction:
C6H5CH2COOAg+Br2CCl4−−→ΔC6H5CH2Br
A
C6H5−CH2−O||C−O.
B
C6H5.CH2
C
Br.
D
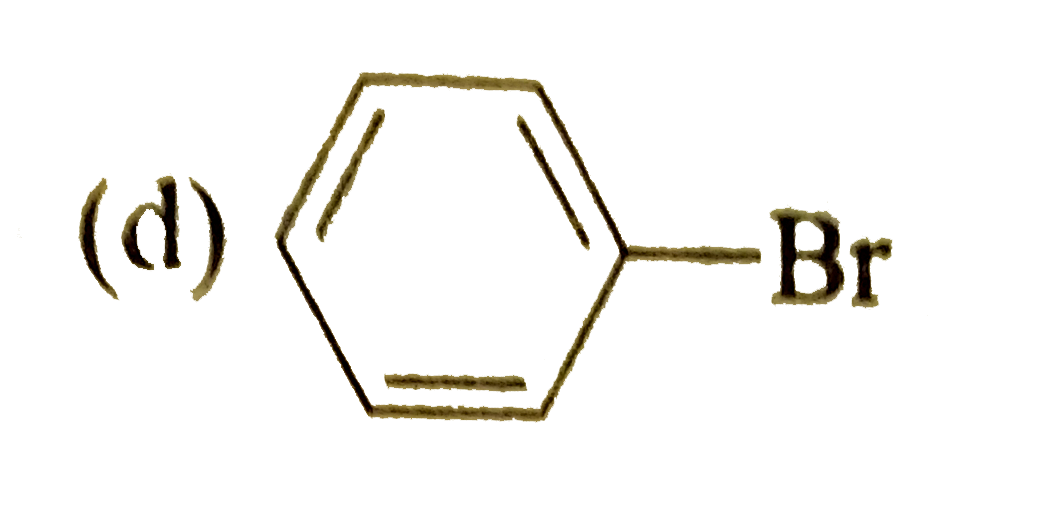
Text Solution
The correct Answer is:A, B, C
|
Similar Questions
Knowledge Check
The intermediate(s) formed during the reaction
C6H5CH2COOAg+Br2CCl4−−→C6H5CH2Br is (are) :AC6H5−CH2−O∣∣OBrBC6H5−CH2−O∣∣∗OCC6H5−.CH2D.BrThe intermediate(s) formed during the reaction
C6C5CH2COOAg+Br2CCl4−−→C6H5CH2Br, is (are):AC6H5−CH2−O∣∣−OBrBC6H5−CH2−O∣∣−∗OCC6H5−∗CH2DB∗rThe intermediates formed during the reaction :
C6H5CH2COOAg+Br2CCl4−−→ΔC6H5CH2BrA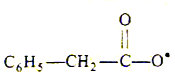 BC6H5.CH2CBr∗D
BC6H5.CH2CBr∗D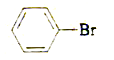
In the given reaction
C6H5−O∣∣C−CH3[X]−−→C6H5−CH2−CH3
[X] will be -
View SolutionC6H6+CH3Clanhydrous−−−−−→AlCl3C6H5CH3+HCl is an example of :
View SolutionThe major end product B in the reaction
C6H5CH2BrAgNO2−−−→A1.LiAIH4−−−−−→2.H3O+B is .
View SolutionWrite the major product of the following reaction C6H5CH2CH3Br2/Δ−−−−→NaCN
View SolutionThe major product of the following reaction is:
C6H5CH2−CH3∣C∣Br−CH2−CH3C2H5Na−−−−→C2H5OH
View Solution

