The dipole moment of 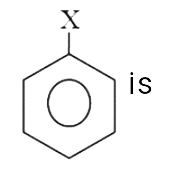 is 1.5 D.
is 1.5 D.
The dipole moment of 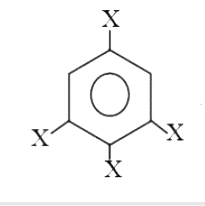 is
is
A
1 D
B
1.5 D
C
2.25 D
D
3 D
Video Solution
Text Solution
The correct Answer is:B
|
Answer
Step by step video, text & image solution for The dipole moment of is 1.5 D. The dipole moment of is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Dipole momentof
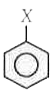 is 1.5 D. The dipole moment of
is 1.5 D. The dipole moment of
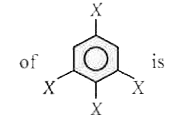 is A1.5 DB2.25 DC1DD3 D
is A1.5 DB2.25 DC1DD3 DDipole moment of
 is 1.5D. The dipole moment of
is 1.5D. The dipole moment of
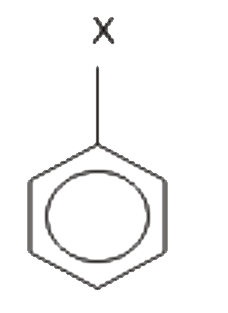
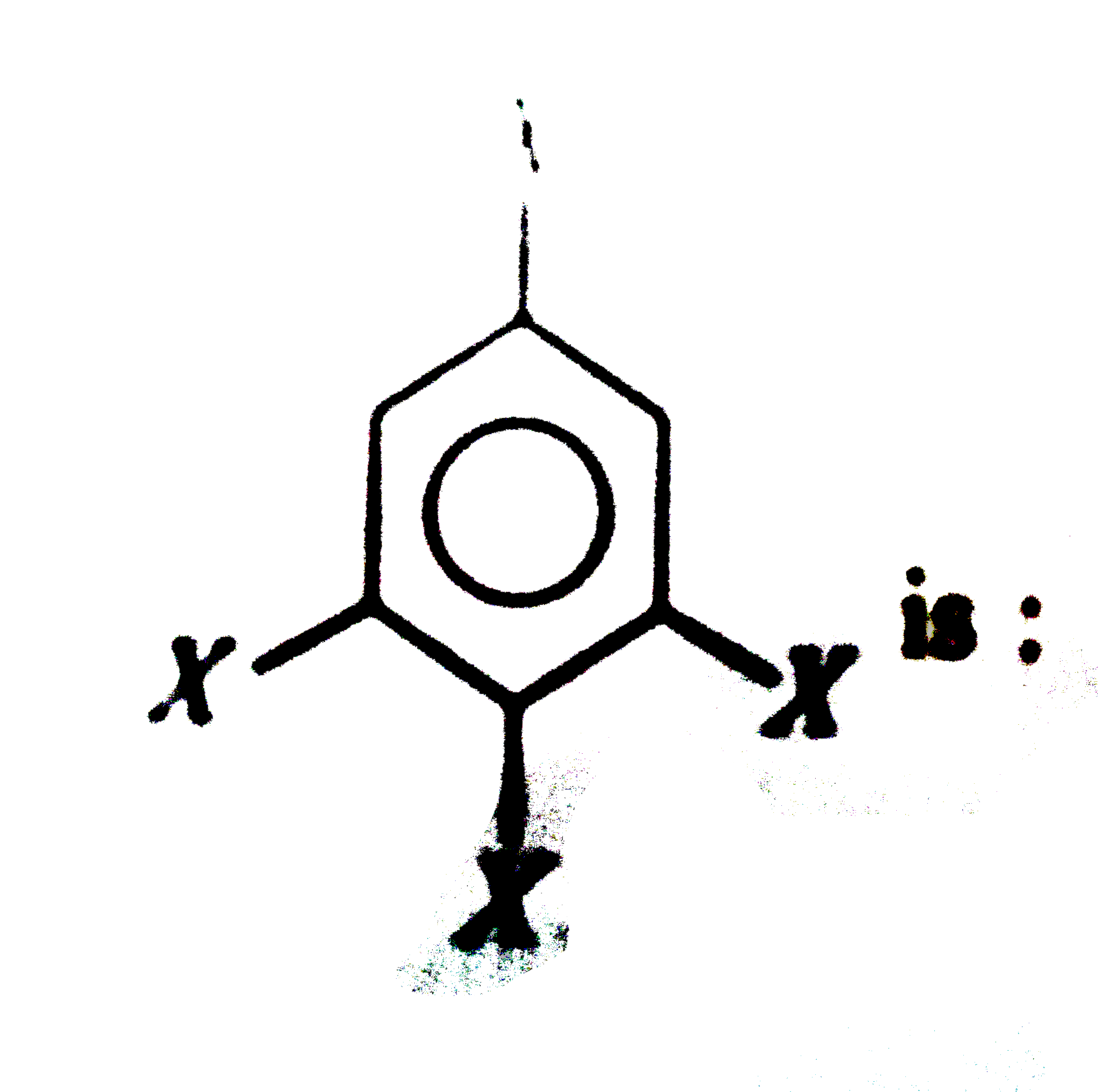 A1DB1.5DC2.25DD3D
A1DB1.5DC2.25DD3DDipole moment of
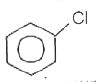 is 1.1 D hence dipole moment of given compound will be
is 1.1 D hence dipole moment of given compound will be
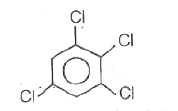 A1.1 DB4.4 DC3.3 DD2.56 D
A1.1 DB4.4 DC3.3 DD2.56 D
An electric dipole is kept on the axis of a uniformly charged ring at distance d from the centre of the ring. The direction of the dipole moment is along the axis. The dipole moment is p, charge of the ring is Q & radius of the ring is R. The force on the dipole is
View SolutionThe dipole moment of chlorobenzene is 1.73 D . The dipole moment of p-dichlorobenzene is expected to be
View SolutionElectric dipole moment is
View SolutionElectric dipole moment is
View SolutionMagnetic dipole moment is a
View Solution

 is 1.5 D.
is 1.5 D. 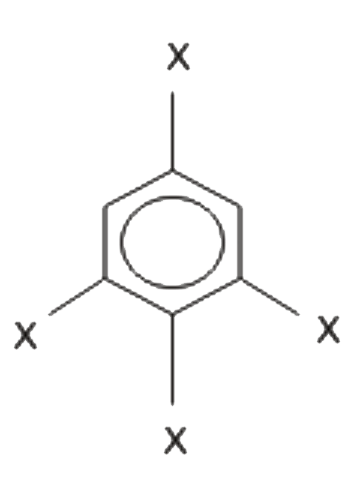 in Debye
in Debye is 1.5D what will be the dipole moment of
is 1.5D what will be the dipole moment of 