The type of overlap given below do not involve in bond formation - Why ?

Text Solution
In gigure I the net overlap becomes zero, since ++ overlap is equal to +− overlap.
In figure II no overlap occurs due to different symmetry.
|
Similar Questions
Which type of overlapping reusults in the formation of a π bond?
View SolutionWhy does type of overlap given in the following figure not result in bond formation?
View SolutionWhy does type of overlap given in the following figure not result in bond formation?
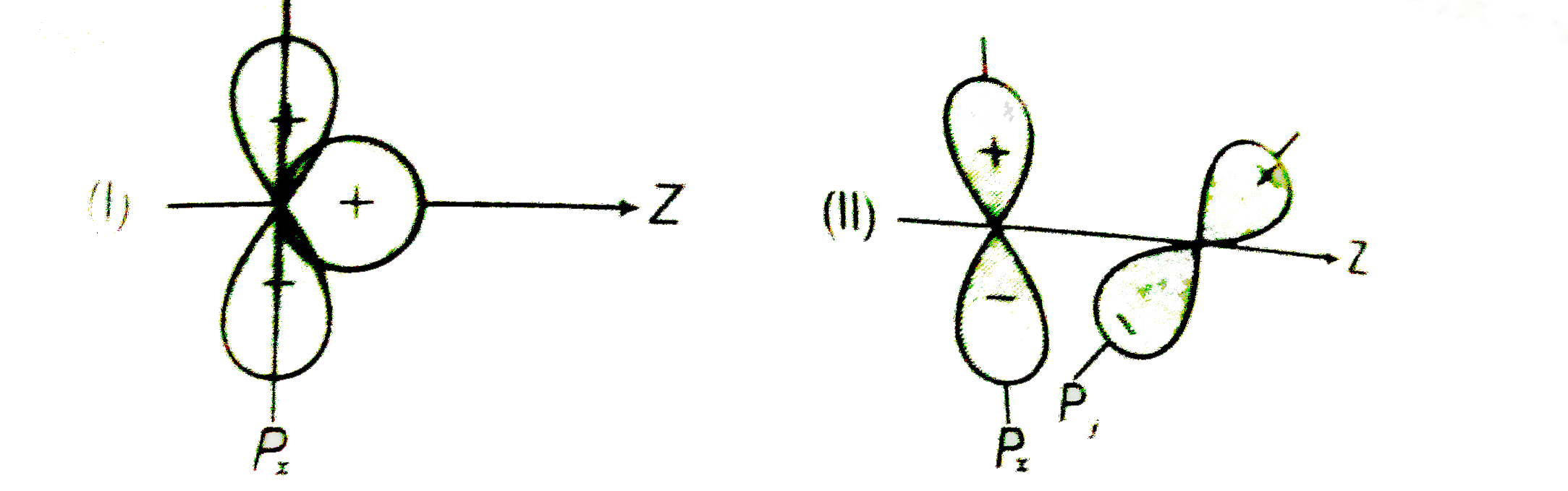
View SolutionWhy does type or overlap given in the following figure not result in bond formation ?
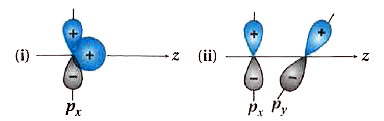
View SolutionCovalent bond formation in H2 involves ________ overlap.
View SolutionWhich type of overlapping reusults in the formation of a π bond?
View SolutionValence Bond Theory || Concept OF Overlapping Axial Overlapping and Formation OF Sigma Bonds
View SolutionWhy does type of overlap given in the following figure not result in bond formation ?
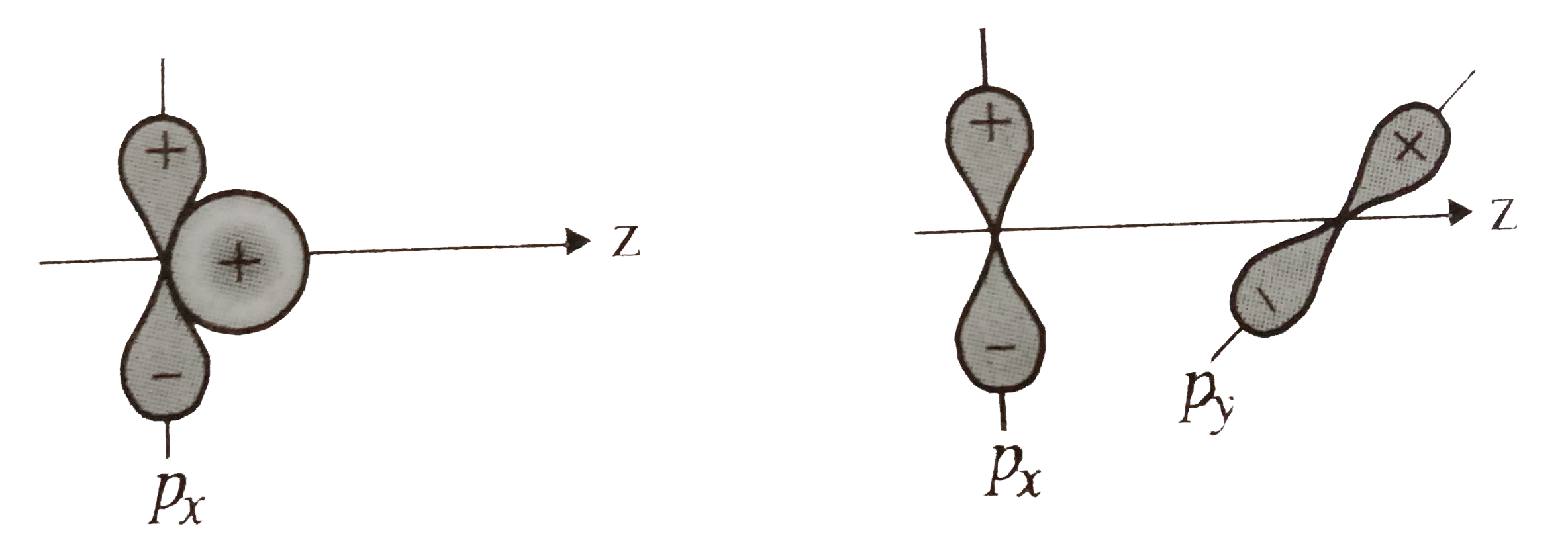
View Solution

