Consider the following reactions,
S(s)+3F2(g)⇔SF6(g)
In each of the above reaction find out whether you have to increase (or) decrease the volume to increase the yield of the product.
Text Solution
S(s)+3F2(g)⇔SF6(g)
In the above equilibrium increase in pressure favours formation of product hence volume should be decreased.
|
Topper's Solved these Questions
- SURAS MODEL QUSETION PAPER - I Book:SURA PUBLICATIONChapter:SURAS MODEL QUSETION PAPER - I Exercise:PART - C
Explore 10 Videos
- SURA'S. MODEL QUESTION PAPER-3Book:SURA PUBLICATIONChapter:SURA'S. MODEL QUESTION PAPER-3Exercise:PART-D
Explore 21 Videos
- SURAS. MODEL QUESTION PAPER - 2 Book:SURA PUBLICATIONChapter:SURAS. MODEL QUESTION PAPER - 2 Exercise:Part - D (Answer all five questions:)
Explore 15 Videos
Similar Questions
Knowledge Check
Consider the following reaction
Phenol+CH2(excess)+H2SO4)→
The expected product(s) of the above reaction is/areA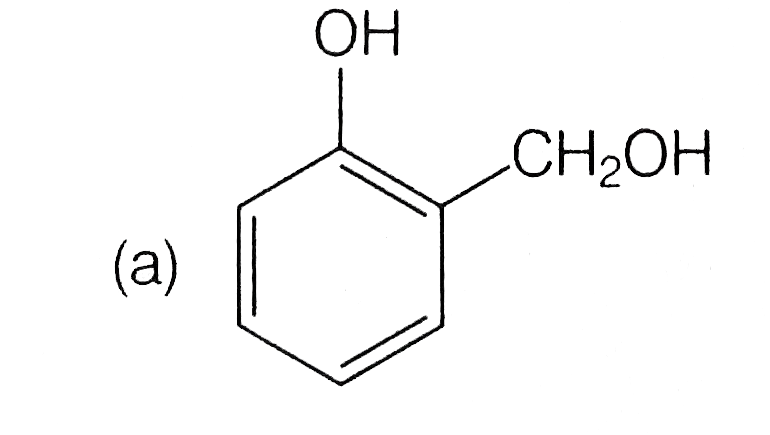 B
B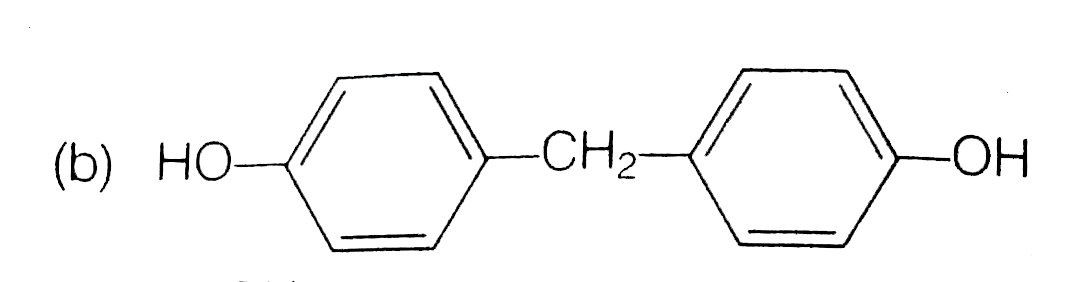 C
C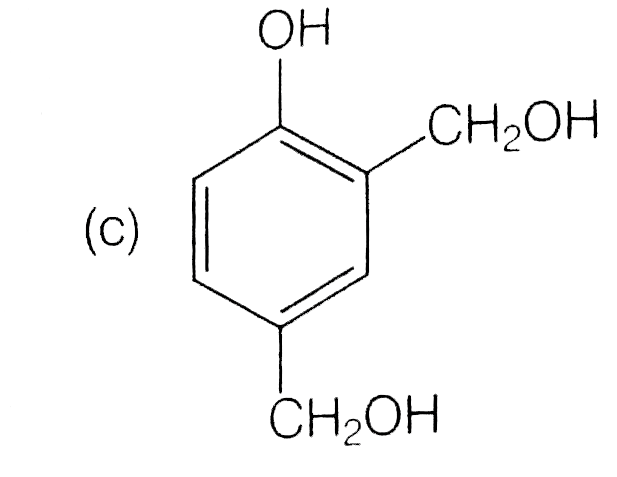 D
D
In which of the following reaction, the yield of the products does not increase by increase in thepressure?
AN2(g)+O2(g)=2NO(g)B2SO2(g)+O2(g)=2SO3(g)CN2(g)+3H2(g)=2NH3(g)DPCl3(g)+Cl2(g)=PCl5(g)2SO2(g)+O2(g)⇔2SO3(g)+Q KJ
In the above reaction, how can the yield of product be increased without increasing the pressure ?Aby increasing temperatureBby decreasing temperatureCby increasing th volume of the reaction vesselDby the addition of the catalyst
Justify that the following reactions are redox reactions :
2K(s)+F2(g)→2K+F−(s)
View SolutionWould you expect equilibrium constant for the reaction l2(g)⇔2l(g) to increase or decrease as temperature increases. Assign reson.
View SolutionWould you expect equilibrium constant for the reaction I2(g)⇔2I(g) to increase or decrease as temperature increases. Assign reson.
View SolutionConsider the following reactions and answer A and B.
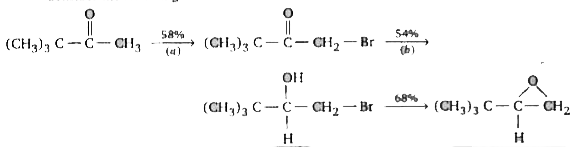
Yield of each step as actually carried out in laboratory is given above each arrow. What is overall yield of the reaction ?
View SolutionBurning of coal is represented as C(s)+O2(g)→CO2(g). The rate of this reaction is increased by
View Solution

