For photoelectric effect in sodium, the figure shows the plot of cut-off voltage versus frequency of incident radiation. Calculate
threshold frequency
The threshold frequency is the frequency of incident light at which kinetic enegy of ejected photoelectrons is zero.
∴ From figure threshold frequency.
V0=4.5×1014Hz.
Similar Questions
For photoelectric effect in sodium, fig. shows the plot of cut-off voltage versus frequency of the incident radiation. Caclculate:
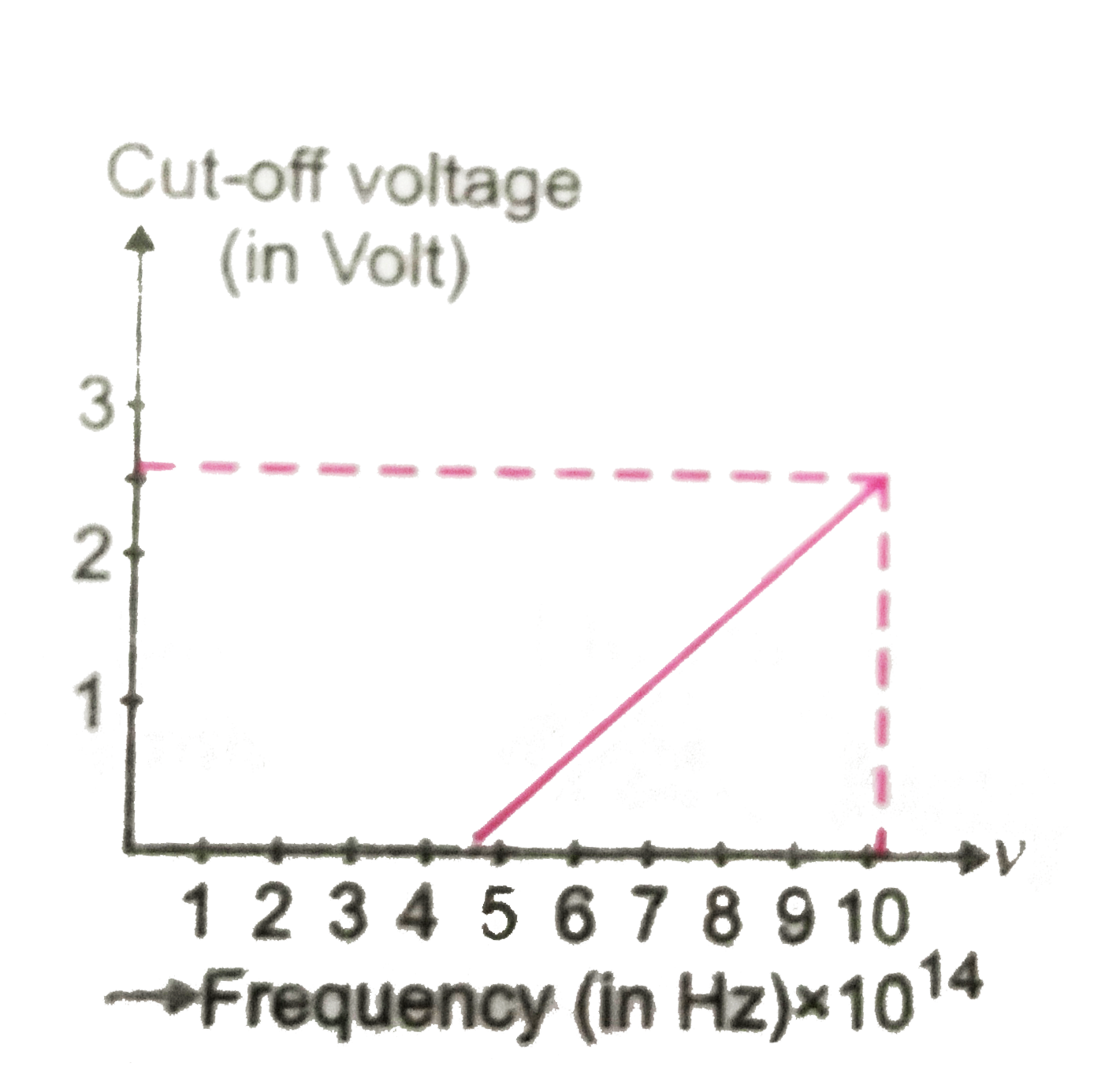
(i) the threshold frequency
(ii) the work function for sodium. (Use h=6.6×10−34Js)
View SolutionIn an experiment on photoelectric effect, the slope of the cut-offf voltage versus frequency of incident light is found to be 4.12×10−15V s. calculate the value of Planck's constant.
View Solution
Knowledge Check
For a photoelectric cell, the graph shown the variation of cut off voltage (V0) with frequency (v) of incident light is
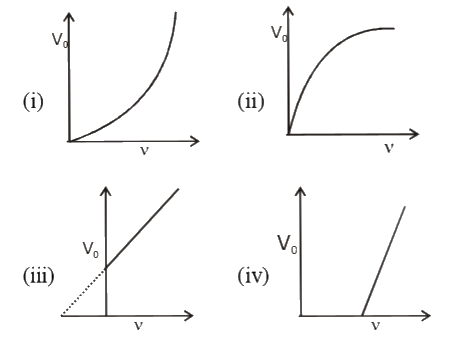 A(i)B(ii)C(iii)D(iv)
A(i)B(ii)C(iii)D(iv)In an experiment on the photoelectric effect , the slope of the cut - off voltage versus frequency of incident light is found to be 4.12×10−15V s . The value of Planck's constant is
A6.592×10−34 J sB6.592×10−31 J sC9.592×10−34 J sD6.592×10−30 J sFor a photoelectric cell the graph showing the variation of cut of voltage (V) with frequency (v) of incident light is best represented by
A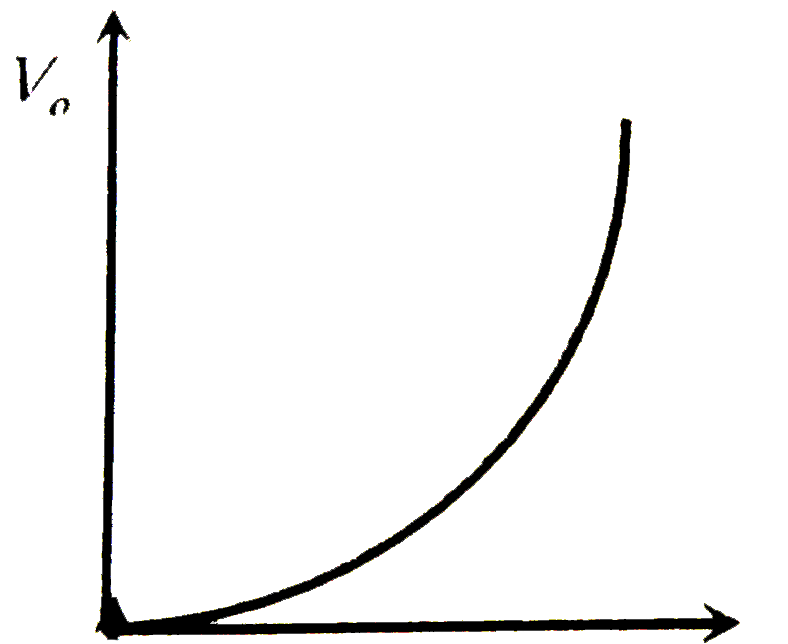 B
B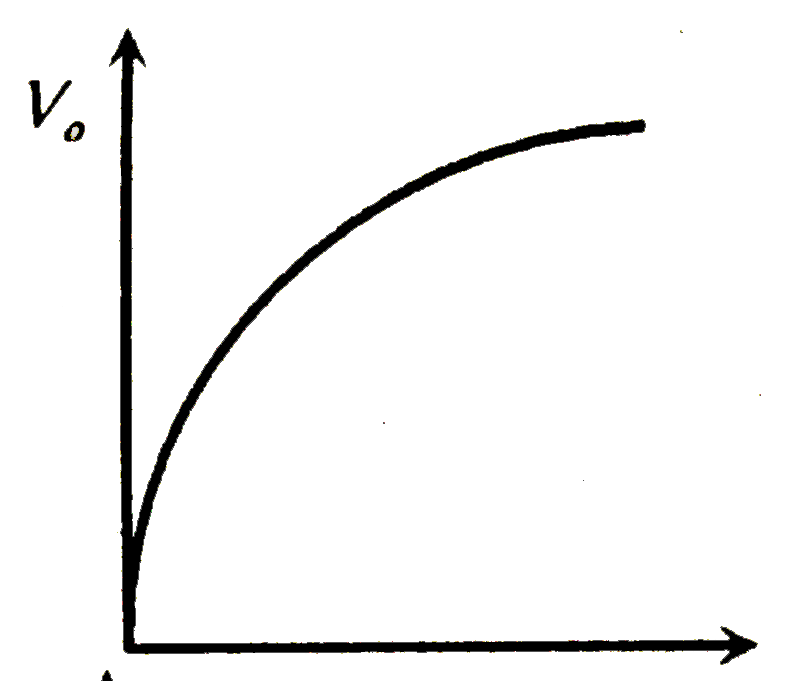 C
C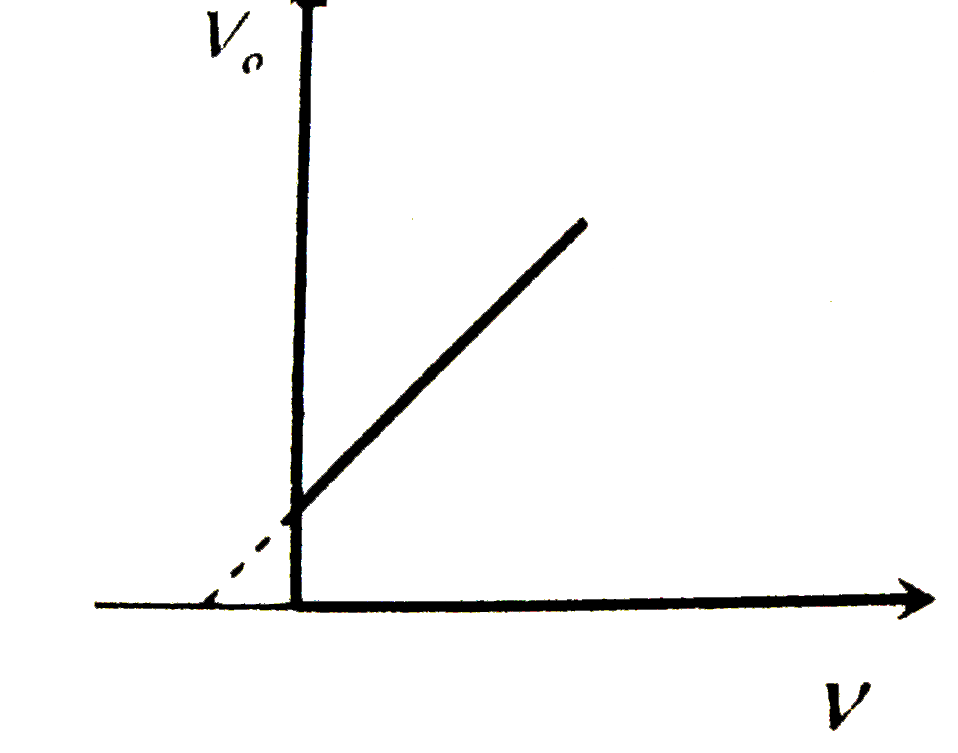 D
D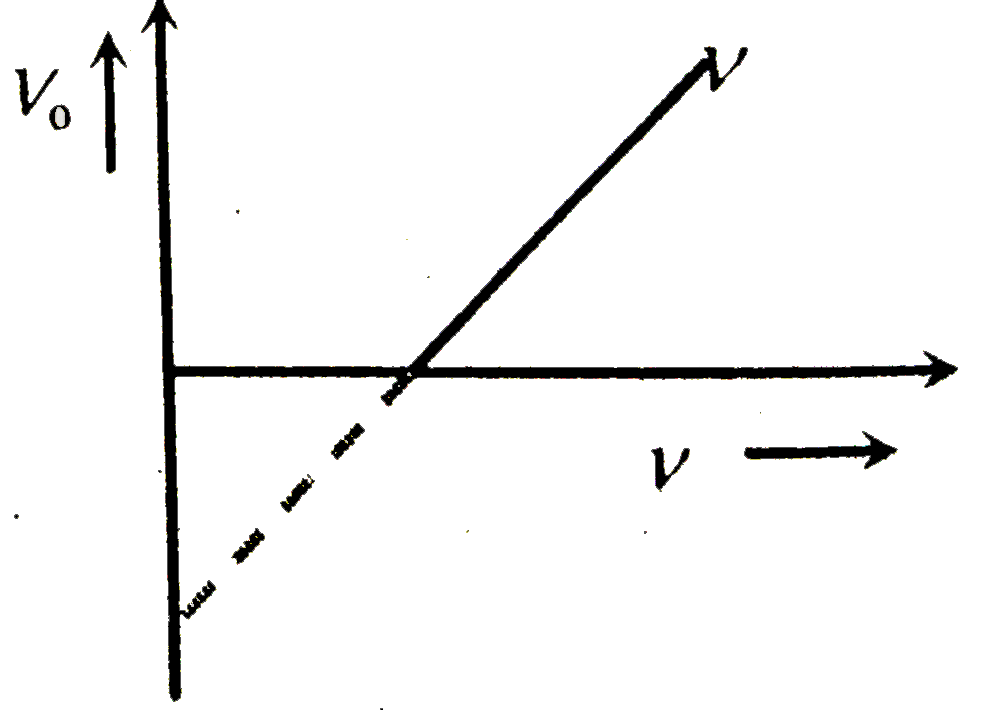
Find a relation between cut-off potential, frequency of the incident light and threshold frequency.
View SolutionIn photoelectric effect the slope of stop of stopping potential versus frequency of incident light for a given surface will be
View SolutionAssertion : The threshold frequency of photoelectric effect supports the particle nature of light.
Reason : If frequency of incident light is less than the threshold frequency, electrons are not emitted from metal surface.
View SolutionAssertion : The threshold frequency of photoelectric effect supports the particle nature of sunlight .
Reason : If frequency of incident light is less than the threshold frequency , electrons are not emitted from metal surface.
View SolutionAssertion : The threshold frequency of photoelectric effect supports the particle nature of light.
Reason : If frequency of incident light is less than the threshold frequency, electrons are not emitted from metal surface.
View Solution

