Match the column I and II
Column (I)Column (II)GroupEffect on phenyl ring(a)−CH=CH−CO2H(p)p/p−directors(b)−O−O∣∣S−CH3(q)meta-directors(c)−NH−O∣∣C−CH3(r)Activating group(d)−S∣∣O−CH3(s)De-activating group
The correct Answer is:a -p, s; b -p, r; c - p, r; d- p, s
Basic information
Topper's Solved these Questions
- AROMATIC COMPOUNDSBook:MS CHOUHANChapter:AROMATIC COMPOUNDSExercise:Subjective Problems
Explore 3 Videos
- AROMATIC COMPOUNDSBook:MS CHOUHANChapter:AROMATIC COMPOUNDSExercise:Solved Problem
Explore 48 Videos
- AROMATIC COMPOUNDSBook:MS CHOUHANChapter:AROMATIC COMPOUNDSExercise:Level 1 (Q.181 To Q.210)
Explore 1 Video
- AN INTRODUCTION TO ORGANIC REACTIONS AND THEIR MECHANISMS ACIDS AND BASESBook:MS CHOUHANChapter:AN INTRODUCTION TO ORGANIC REACTIONS AND THEIR MECHANISMS ACIDS AND BASESExercise:ADDITIONAL QUESTION (SINGLE CORRECT CHOICE TYPE )
Explore 32 Videos
- BIOMOLECULESBook:MS CHOUHANChapter:BIOMOLECULESExercise:Level 2
Explore 1 Video
Similar Questions

Double bond equivalent of D is:
View SolutionProvide a name for each of the following compounds.
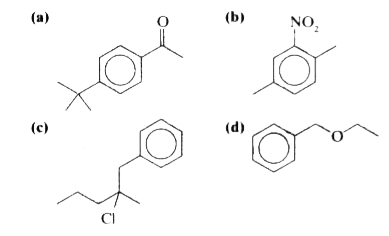
View SolutionProduct obtained in the following transformation is:
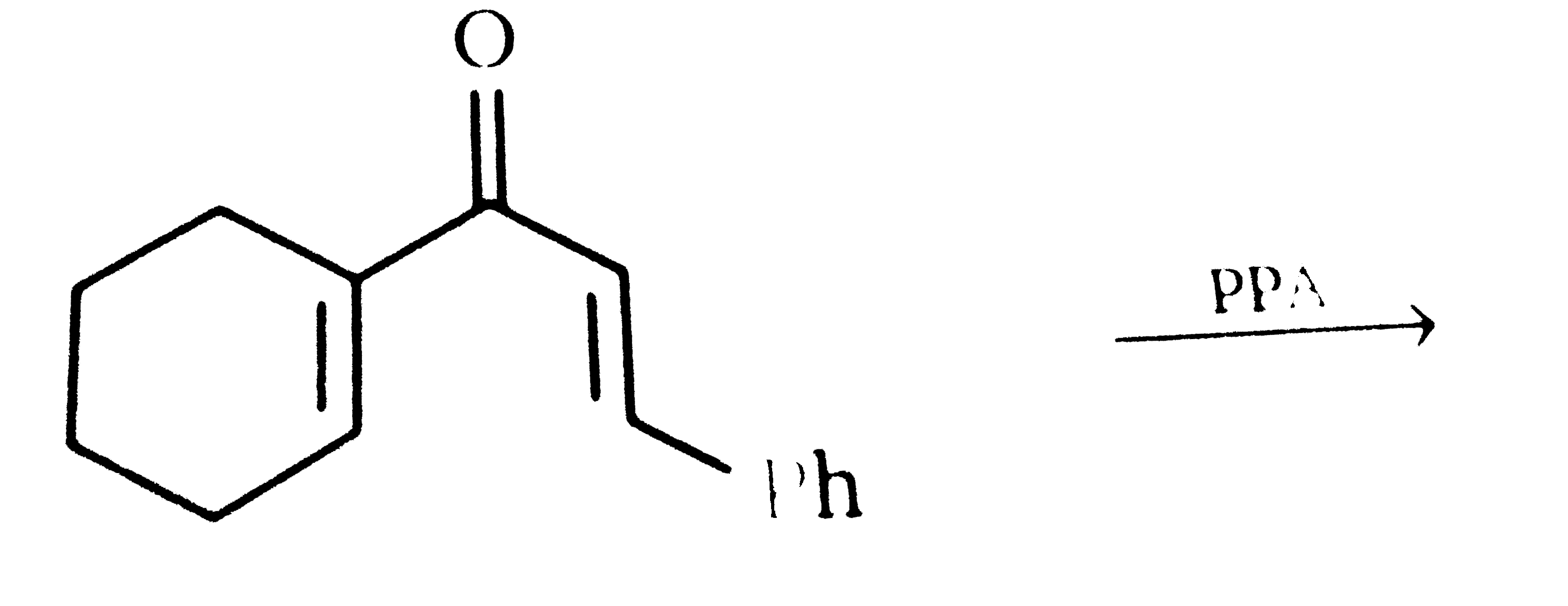
View Solution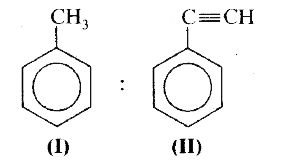
In compound (I) and (II) inductive effect of phenyl is
View SolutionHow many compound which is given below is isomer of D-Glucose ?
D-Mannose, D-Fructose, D-Gulose, D-Idose, D-Galactose, D-Arabinose, D-Ribose.
View SolutionMatch the column
Column I(Reaction)Column II(Number of functional group in product)(A). GlucoseHI−−→(P). 2(B). GlucoseBr2water−−−−→(Q). 3(C). GlucoseHCN−−→(R). 0(D). GlucoseCH3−O∣∣C−O−O∣∣C−CH3−−−−−−−−−−−−−−→(excess)(E). GlucoseHNO3−−−→
View SolutionCH_(3)-underset(CH_(3))underset(|)(CH)-CH=CH-CH_(2)-overset(O)overset(||)(C)-OH
View SolutionChoose the correct case of hydrolysis for the following molecules : (P) CH_(3)-overset(O)overset("||")C-OCPh_(3) (Q) CH_(3)-underset(CH_(3))underset(|)CH-overset(O)overset("||")C-O-CPh_(3) (R) Ph-underset(CH_(3))underset(|)CH-overset(O)overset("||")C-O-CPh_(3) (S) CH_(3)CH_(2)-underset(CH_(3))underset(|)CH-overset(O)overset("||")C-O-CPh_(3)
View SolutionWhen given substituents on a benzene ring, as activating or de-activating and as ortho-para or meta directing for electrophilic aromatic substitution fill the following by appropriate right or wrong. {:(,"Substituent","Activating","De-activating","Ortho/para","Meta",),(1,-OCH_(3),,,,,),(2,-overset(O)overset(||)(C)-O-CH_(3),,,,,),(3,-O-underset(O)underset(||)(C)- CH_(3),,,,,),(4,-CH_(3),,,,,),(5,-F,,,,,),(6,-Ph,,,,,),(7,-NH - overset(O)overset(||)(C) - CH_(3),,,,,),(8,-overset(O)overset(||)(C) - NH - CH_(3),,,,,),(9,-Br,,,,,),(10,-CN,,,,,),(11,-CF_(3),,,,,),(12,-overset(O)overset(||)(C) - NH_(2),,,,,),(13,-overset(O)overset(||)(C) - OH,,,,,),(14,-CH = CH_(2),,,,,),(15,-CH=CH-overset(O)overset(||)(C) - OH,,,,,),(16,-CH = CH - overset(O)overset(||)(C) - H,,,,,),(17,-S-Et,,,,,),(18,-underset(O)underset(||)(S)- Et,,,,,),(19,-underset(O)underset(||)overset(O)overset(||)(S) - Et,,,,,),(20,-N = O,,,,,),(21,-CH_(2)X,,,,,),(22,-CHX_(2),,,,,):}
View SolutionArrange the following anions in decreasing order of stability :
H-overset(O)overset(||)C-underset((P))overset(Ɵ)CH-overset(O)overset(||)C-H
CH3−O∣∣C−ƟC(Q)H−O∣∣C−H
CH3−O∣∣C−ƟCH−(R)O∣∣C−⋅⋅O⋅⋅−CH3
View SolutionColumn(I)Column(II)(a)CH3−⊕CH−CH3P.Pyramidal structure(b)ΘCH3Q.Planar geometry(c)CH3−.CH−CH3R.Electrophile(d)Singlest carbeneS.Nucleophile
View SolutionCH_(3)underset(CH_(3))underset(|)overset(CH_(3))overset(|)C-CH=CH_(2)overset(overset(+)(H)|H_(2)o)(to)'P' (major) product 'P' is-
View SolutionIn the following sets of resonating structure, lable the major and minor contributors towards resonance hybrid. (P)underset("(I)")(CH_(3)-overset(Theta)CH-C-=N)harrunderset("(II)")(CH_(3)CH=C=overset(..)N:^(Theta))" (Q)"underset("(I)")(CH_(3)-overset(Theta)CH-CH=CH-NO)harrunderset("(II)")(CH_(3)-CH=CH-CH=overset(o+)underset(O^(Theta))underset(|)N-O^(Theta)) (R)underset("(I)")(CH_(3)-CH_(2)-overset(o+)underset(""NH_(2))underset(|)(C)-NH_(2))harrunderset("(II)")(CH_(3)-CH_(2)-underset(""NH_(2))underset(|)(C)=overset(o+)NH_(2))" (S)"underset("(I)")(CH_(3)-overset(O)overset(||)C-overset(Theta)CH-overset(O)overset(||)C-CH_(3))harrunderset("(II)")(CH_(3)-overset(O^(Theta))overset(|)C=CH-overset(O)overset(||)C-CH_(3)
View SolutionFollowing is an example of
CH2=CH−O∣∣CH↔⊕CH2−CH=O−| C−H↔.−CH2=CH−O−| C ⊕−H
View SolutionPh−CH=CH−−COH(i)CH3CH2MgBr−−−−−−−−−−−→(ii)H3O⊕−−−−−−→(X)Cu,Δ−−−→(Y)
View Solution

