Calculate packing efficiency in simple cubic unit cell. Solution in Kannada
Video Solution
Text Solution
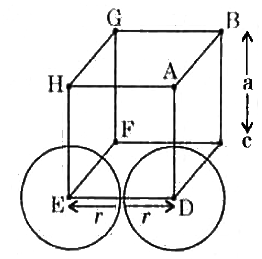
In simple cubic lattice the atoms are located only on the corners of the cube.
(a) The atoms touch each other along the edge.
(b) Edge length of the cube is 'a' and radius of the each atom is r. ∴a=2r.
( c) The volume of unit cell is a3=(2r)3=8r3
( d) Simple cubic unit cell contain only one atom.
( e) The volume of the one atom =43πr3
Packing efficiency =volume of one atomvolume of the cubic unit cell×100
4/3πr38r3×100
=π/6×100=52.4%
|
Similar Questions
Calculate the packing efficiency in a simple cubic lattice.
View SolutionCaleulate the packing efficiency in a unit cell of Cubic Close Packing(CCP) structure.
View SolutionSimple Cubic Unit cell |Packing Efficiency Of SCC #!#Voids in 3D unit cells #!#PYQs
View SolutionExplain packing in simple cubic unit cell crystal.
View SolutionWrite the steps involved to calculate the percentage of efficiency of packing of simple cubic crystal Or packing efficiency for simple cubic unit cell? Or Calculate the paking efficiency of metal crystal that has simple cubic structure?
View SolutionCalculate the packing efficiency in simple cubic unit cell.
View Solutionसरल घनीय एकक कोष्ठिका में संकुलन दक्षता के प्रतिशत की गणना कीजिए।
View SolutionThe efficiency of packing in simple cubic unit cell is
View Solution

