Caleulate the packing efficiency in a unit cell of Cubic Close Packing(CCP) structure. Solution in Kannada
a) Packing efficiency is a percentage of total space filled by the particles in a crystal. The unit cell of a simple cube is
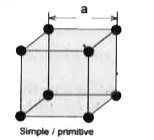
A simple cubic unit cell consists of only one atom/ particle/sphere per unit cell. Let .a. be the edgelength of the unit cell and .r. be the radius of the sphere.
We know that volume of the spherer =43πr3
The spheres/ lattice points at the corners in the simple cubic unit cell are touching each other. Therefore edge length =a=2r
Similarly volume of the cube =a=(2r)3=8r3
Percentage of the space occupied by spheres
=Volume of the sphereVolume of the cube×100=43πr38r3×100
=4×100×228×3×7
=52.38%≈52.4% is the packing fraction in simple cubic lattice
Topper's Solved these Questions
- II PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2015)Book:SUNSTAR PUBLICATIONChapter:II PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2015)Exercise:PART - C
Explore 8 Videos
- II PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2014)Book:SUNSTAR PUBLICATIONChapter:II PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2014)Exercise:PART - D
Explore 22 Videos
- II PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2016)Book:SUNSTAR PUBLICATIONChapter:II PUC CHEMISTRY (ANNUAL EXAM QUESTION PAPER MARCH - 2016)Exercise:PART - D
Explore 26 Videos
Similar Questions
Describe the three steps involved in the leaching of bauxite to get pure alumina (equations not expected).
View SolutionCalculate the packing efficiency in a simple cubic lattice.
View Solutiona. Calculate the packing efficiency in a Body Centered Cubic (BCC) lattice.
b. Silver forms a ccp lattice. The edge length of its unit cell is 408.6 pm. Calculate the density of silver. (NA=6.022×1023, Atomic mass of Ag =108gmol−1)
View SolutionThe correct statement (s) for cubic close packed (ccp) three dimensional structure is (are)
View SolutionIn the cubic close close packing, the unit cell has....
View SolutionWhat is the packing efficiency is ccp structure?
View SolutionThe correct statement (s) for cubic close packed (ccp) three dimensional structure is (are)
View SolutionIn the uint cell of a cubic close-packed struture, total number of voids is ……. Whereas in the unit cell of a hexagonal close-packed stucture, total number is voids is ……
View SolutionThe CORRECT statement for cubic close packed (ccp) three-dimensional structure is (are)
View SolutionWhat is packing efficiency of a unit cell? Show that the packing efficiency of a simple cubic unit cell is 52.4%.
View SolutionWhat is the unit cell in a cubic close-packed (ccp) structure of particles?
View SolutionThe packing efficiency of simple cubic (sc.) body centred cubic (bcc) and cubic close packing (ccp) lattices follow the order
View SolutionThe packing efficiency in hcp and ccp structures is
View Solution(a) Compare the packing efficiency of square close packing, hexagonal close packing and cubic close packing.
(b) Give the values of packing efficiency in (i) hcp or ccp and (ii) body-centred cubic crystals.
View SolutionCaleulate the packing efficiency in a unit cell of Cubic Close Packing(CCP) structure.
View Solution
