Describe the construction and working of standard hydrogen electrode. Solution in Kannada
Video Solution
Text Solution
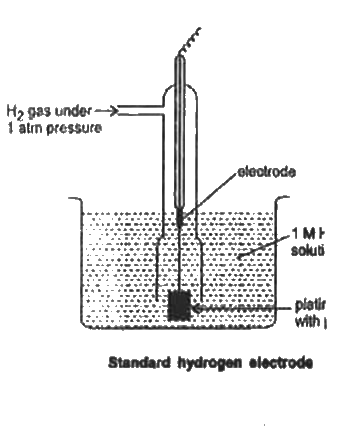
The standard hydrogen electrode consists of a platinum electrode coated with platinum black. The electrode is dipped in an acidic solution containing 1MH+ ions. Pure hydrogen gas at one bar pressure is bubbled through it.
Standard hydrogen electrode (SHE) is represented as
(Pt(s)|H2(g)|H+(aq)
Potential is developed when equillibrium attained between H+ ions of the solution and H2 gas on the interface of the electrode.
H+(aq)+e−→12H2(g)
The electrode potential for standard hydrogen electrode (SHE) is taken zero at all temperature.
|
Similar Questions
Describe the constitution of standard hydrogen electrode.
View SolutionDescribe the construction and working of standard hydrogen electrode.
View SolutionDescribe the construction of standard hydrogen electrode.
View SolutionDescribe the working of standard hydrogen electrode.
View SolutionWhat is the standard hydrogen electrode? Briefly describe the measurement of potential of a single electron.
View SolutionDescribe the construction and working of standard hydrogen electrode.
View SolutionExpress standard Hydrogen electrode.
View SolutionElectrode potential || EMF OF Cell || Standard Hydrogen Electrode || Measurement OF Standard Electrode Potential || Significance OF Standard Electrode Potential
View Solution
