Describe the construction and working of Normal Hydrogen Electrode.
Text Solution
The absolute value of a single electrode potential cannot be determined experimentally because neither the oxidation nor the reduction can occur by itself. Therefore, we can find only the relative electrode potentials. For this purpose, hydrogen electrode has been selected as the reference electrode and is given arbitrary value of zero in its standard state i.e., 2H+(C=1 mol l−1)+2e−→H2(g)(1atm):E∘=0.00V
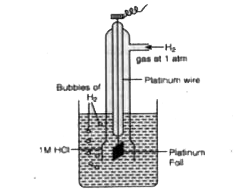
The standard hydrogen electrode consists of platinum wire sealed in a glass tube and has a platinum foil attached to it. The foil is coated with finely divided platinum and acts as platinum electrode. It is dipped into an acid solution containing H+ ions in 1 M concentration (1 M HCI). Pure hydrogen gas at 1 atmospheric pressure is constantly bubbled into the solution at constant temperature of 298 K. The surface of the foil acts as a site for the reaction.
The following reactions occur in this half cell depending upon whether it acts as an anode or as a cathode.
If S.H.E. acts as anode
H2(g)→2H++2e−
If S.H.E. acts as cathode
2H++e−→H2(g)
The electrode potential of an electrode can be measured by connecting this half cell with standard hydrogen electrode, whose potential is taken as zero.
|
Similar Questions
Knowledge Check
Hydrogen electrode is
Areversible with respect to H+ ionsBa secondary reference electrodeCan indicator electrodeDirreversible with respect to H+ ions
Describe the construction and working of the calomel electrode.
View SolutionWhat is a primary cell ? Describe the construction and working of dry cell,
View SolutionVan de Graff generator is used to produce high energetic charged particles of energy of about
View SolutionDescribe the construction and working of a magnetic compass.
View SolutionDescribe the construction and working of compound microscope.
View SolutionGive the construction and working of a solar cooker.
View SolutionGive the construction and working of a solar cooker.
View Solution

