Hyperconjugation occurs in :
None of these
The correct Answer is:C
To determine where hyperconjugation occurs among the given options, we need to analyze the structures of each compound and identify the presence of carbocations and alpha hydrogens. Hyperconjugation involves the interaction of the electrons in a sigma bond (usually C-H or C-C) with an adjacent empty or partially filled p-orbital or π-bond.
1. Understanding Hyperconjugation:
- Hyperconjugation occurs when there is a carbocation (positively charged carbon) adjacent to an sp³ hybridized carbon. The hydrogen atoms attached to this sp³ carbon are called alpha hydrogens and can stabilize the carbocation through hyperconjugation.
2. Analyzing Each Option:
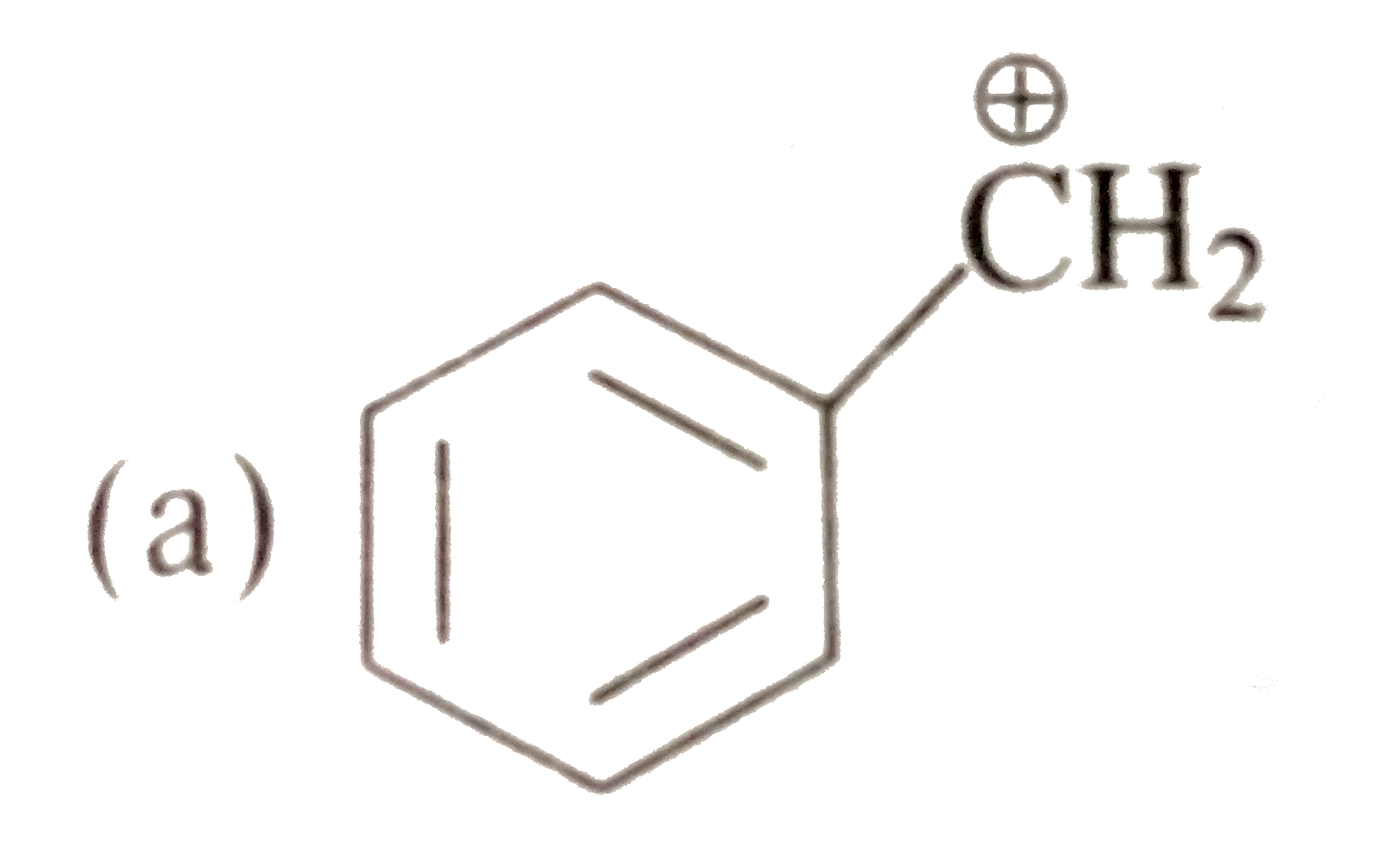
- Option A: Check for a carbocation and adjacent sp³ carbons.
- There is no sp³ hybridized carbon adjacent to a carbocation. Thus, hyperconjugation does not occur here.
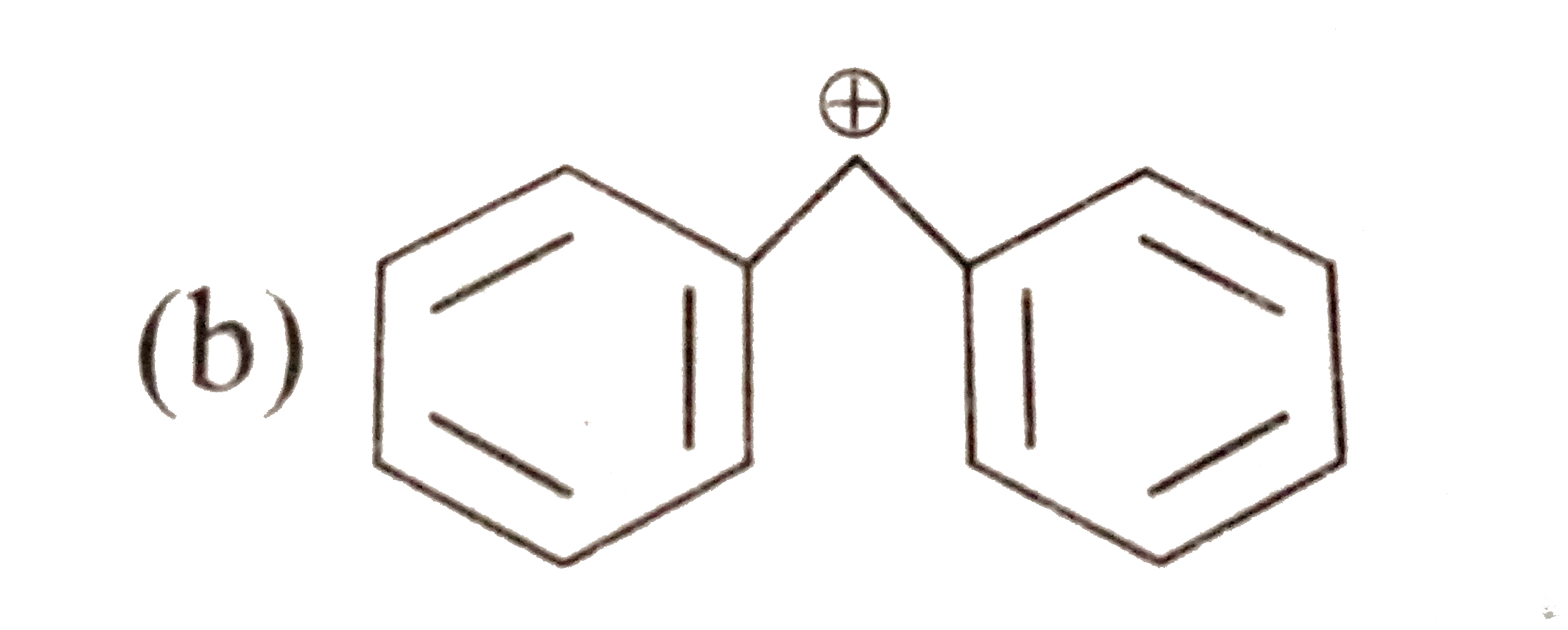
- Option B: Check for a carbocation and adjacent sp³ carbons.
- Similar to option A, there is no sp³ hybridized carbon adjacent to a carbocation. Therefore, hyperconjugation is not present.
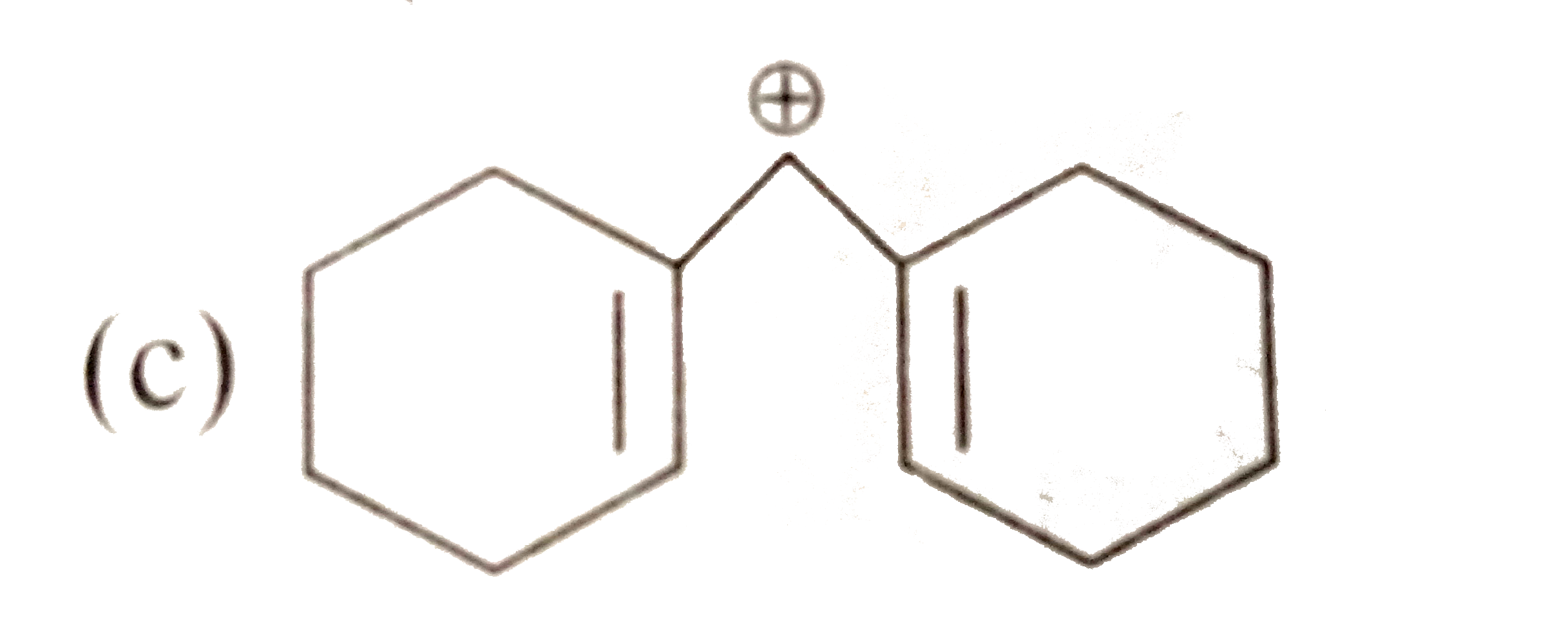
- Option C: Check for a carbocation and adjacent sp³ carbons.
- Here, we can identify a carbocation. If we shift the double bond, we can generate a carbocation on an sp³ hybridized carbon. This sp³ carbon has alpha hydrogens that can participate in hyperconjugation. Thus, hyperconjugation occurs in this option.
3. Conclusion:
- Based on the analysis, hyperconjugation occurs in Option C due to the presence of an sp³ hybridized carbon adjacent to a carbocation that has alpha hydrogens.
Final Answer:
Hyperconjugation occurs in Option C.
- Hyperconjugation occurs when there is a carbocation (positively charged carbon) adjacent to an sp³ hybridized carbon. The hydrogen atoms attached to this sp³ carbon are called alpha hydrogens and can stabilize the carbocation through hyperconjugation.
- Option A: Check for a carbocation and adjacent sp³ carbons.
- There is no sp³ hybridized carbon adjacent to a carbocation. Thus, hyperconjugation does not occur here.
- Option B: Check for a carbocation and adjacent sp³ carbons.
- Similar to option A, there is no sp³ hybridized carbon adjacent to a carbocation. Therefore, hyperconjugation is not present.
- Here, we can identify a carbocation. If we shift the double bond, we can generate a carbocation on an sp³ hybridized carbon. This sp³ carbon has alpha hydrogens that can participate in hyperconjugation. Thus, hyperconjugation occurs in this option.
- Based on the analysis, hyperconjugation occurs in Option C due to the presence of an sp³ hybridized carbon adjacent to a carbocation that has alpha hydrogens.
Hyperconjugation occurs in Option C.
Similar Questions
Knowledge Check
Hyperconjugation is
Aσ−π conjugationBnoticed due to delocalisation of σandπ bondCno bond resonanceDall the aboveHyperconjugation occur due to overlaping of
Aπ M.O with 's'Bp' orbital with σCσ M.O. with π M.O.DNoneHyperconjugation observed in
A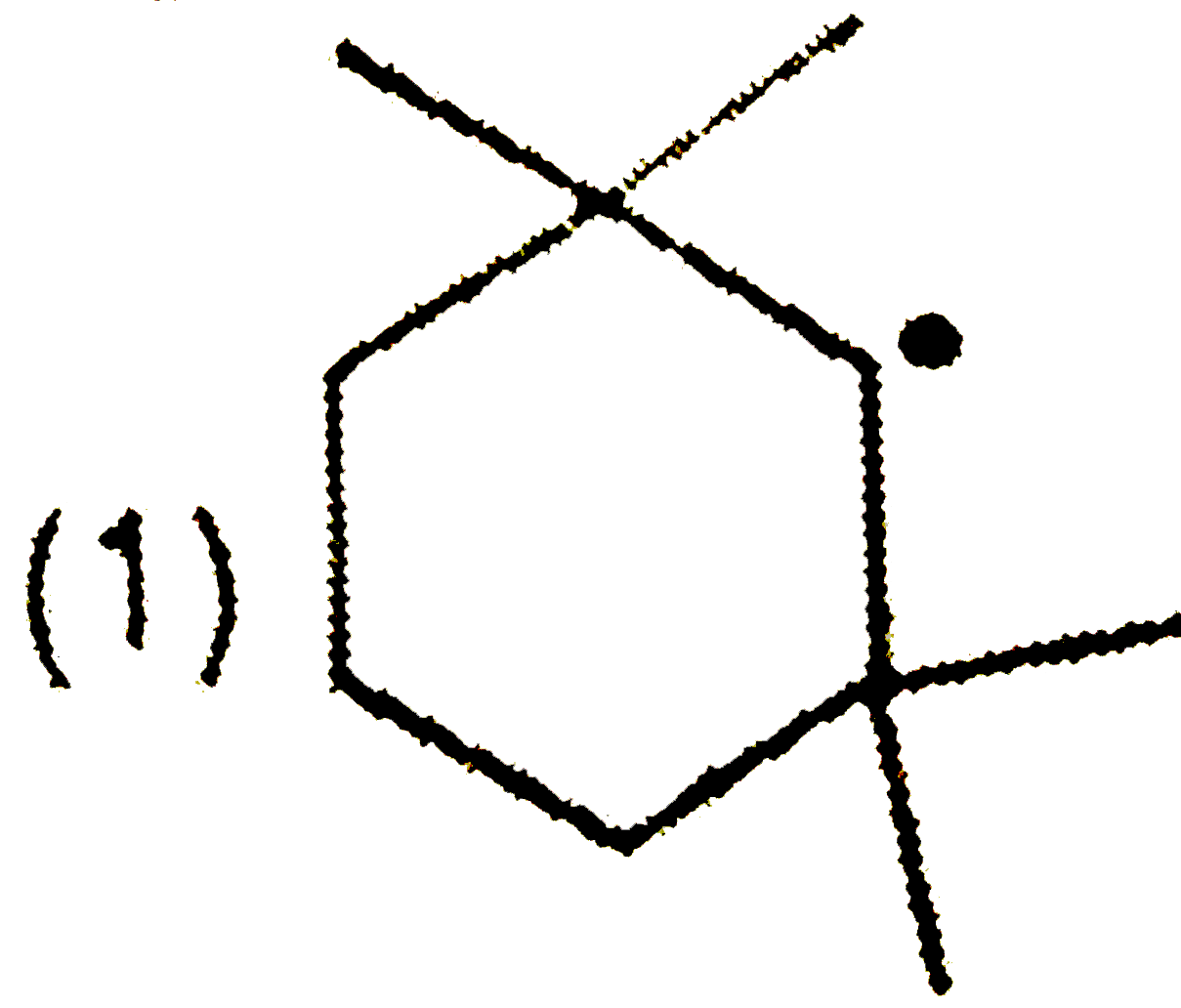 B.CPh3C
B.CPh3C D
D
Hyperconjugation in Alkenes|| Stability OF Alkenes|| Heat OF Hydrogenation|| Heat OF Combustion||Hyperconjugation in Carbocation and Stability Comparison||Hyperconjugation in Free Radicals and stability comparison
View Solution|| Hyperconjugation (+H and -H effect) ||
View SolutionHyperconjugation involves
View SolutionHyperconjugation is not possible in
View SolutionHyperconjugation is more pronounced in
View Solution
