Which of the following aromatic rings have greater electron density than
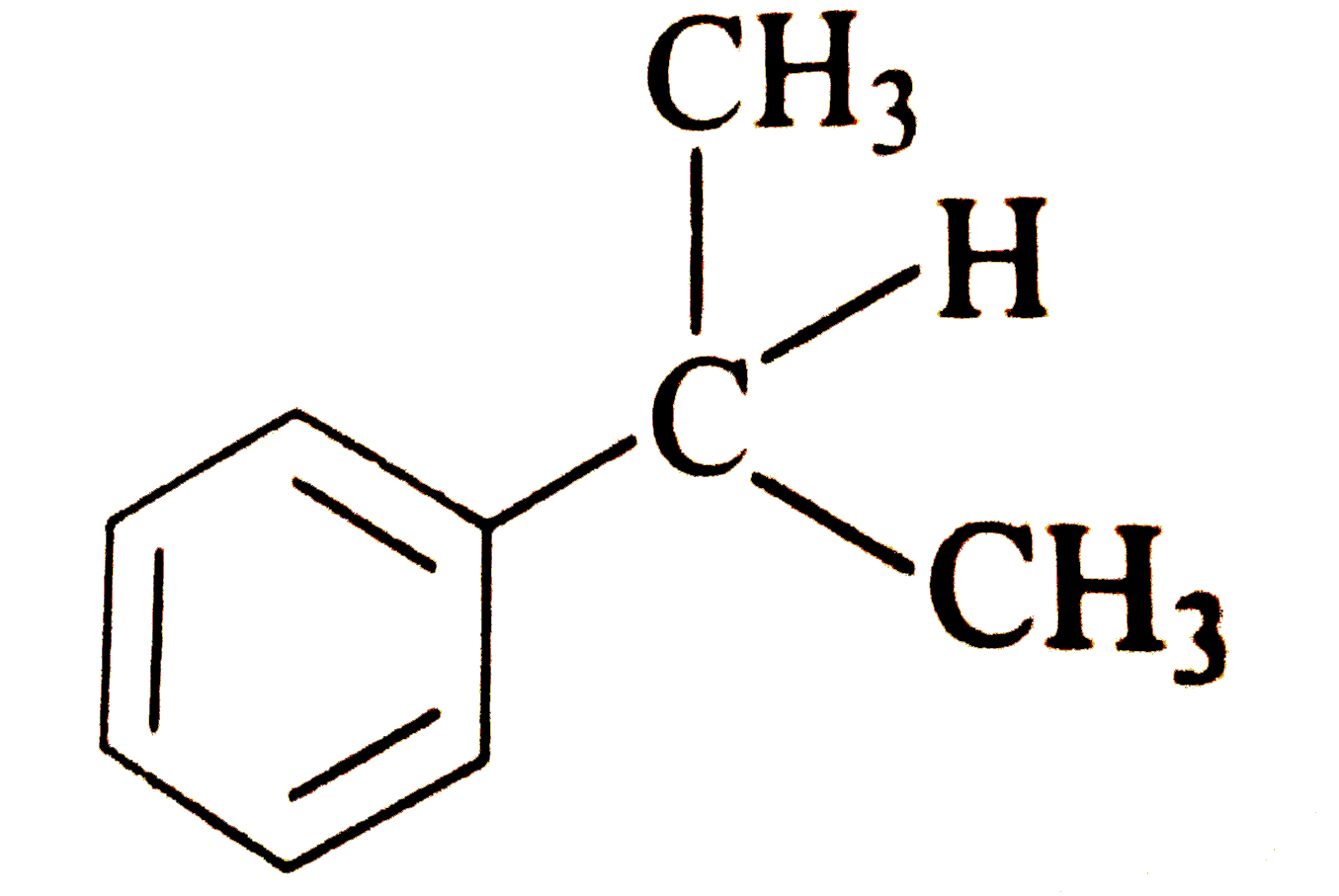
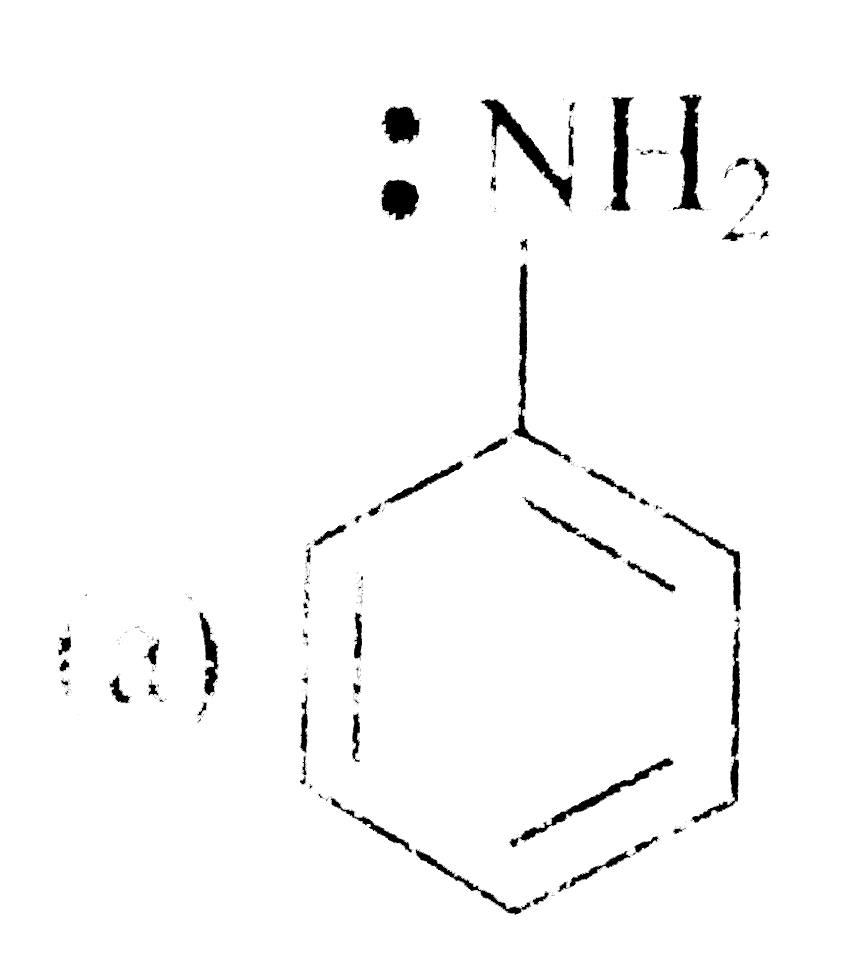
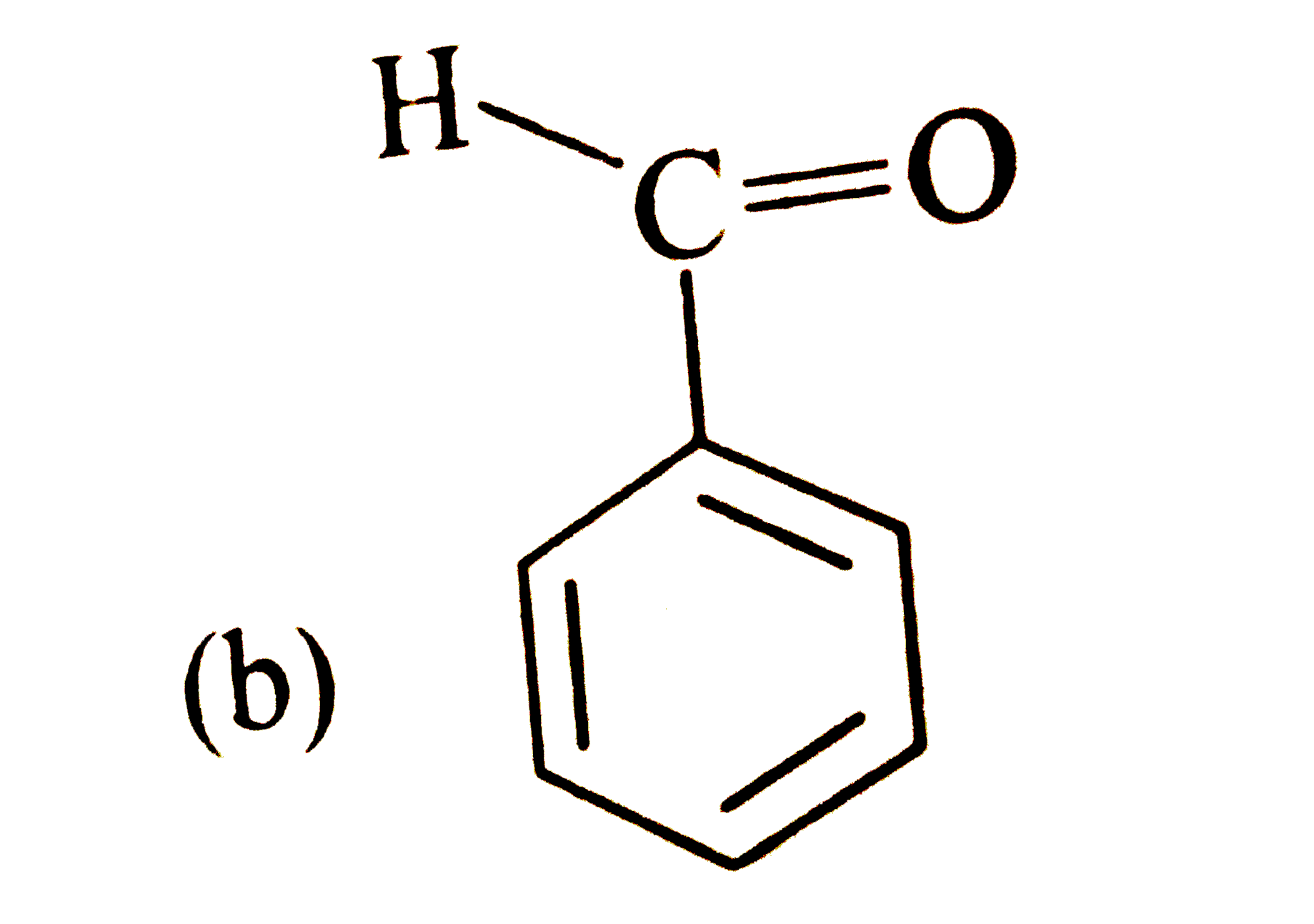
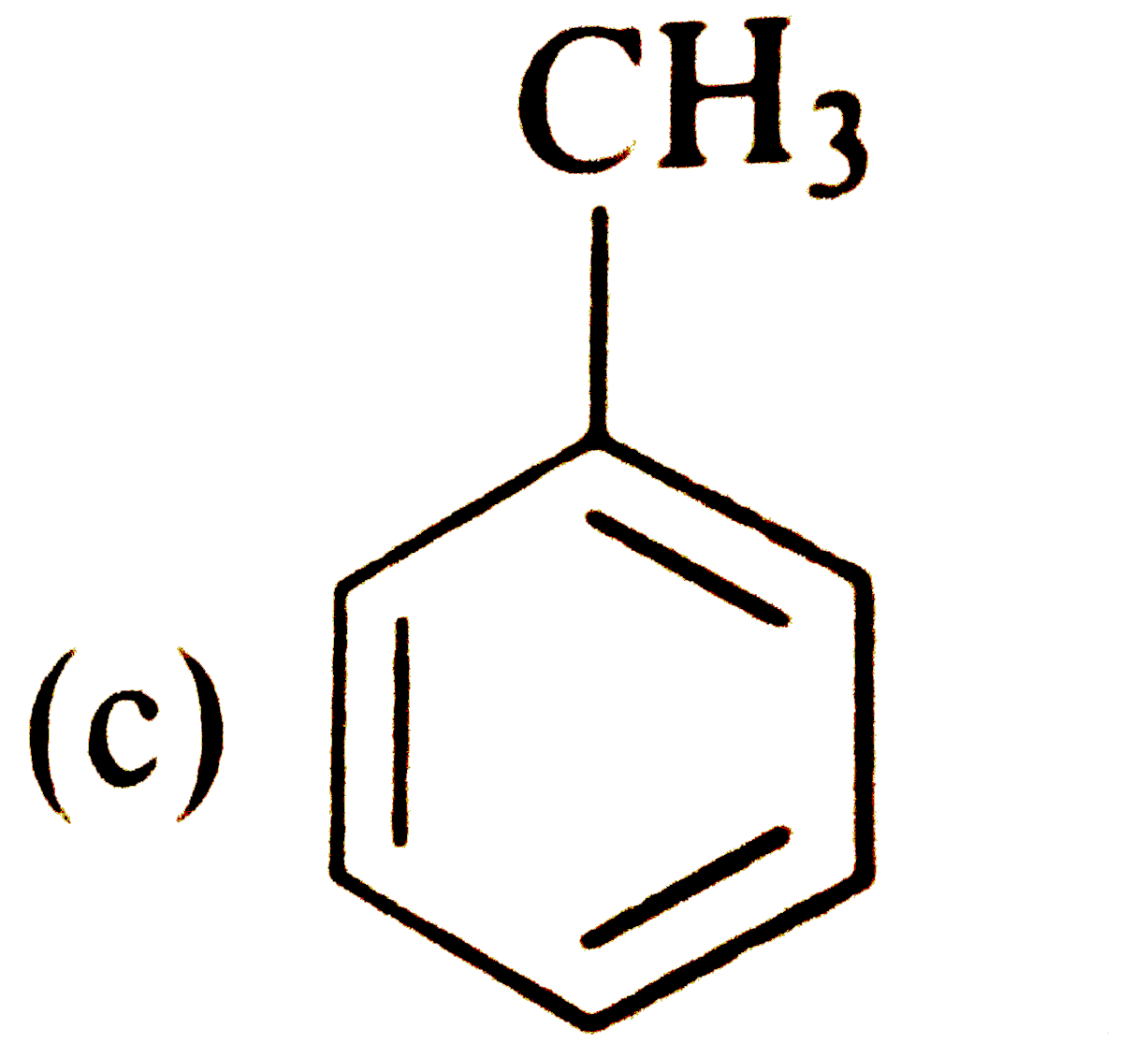
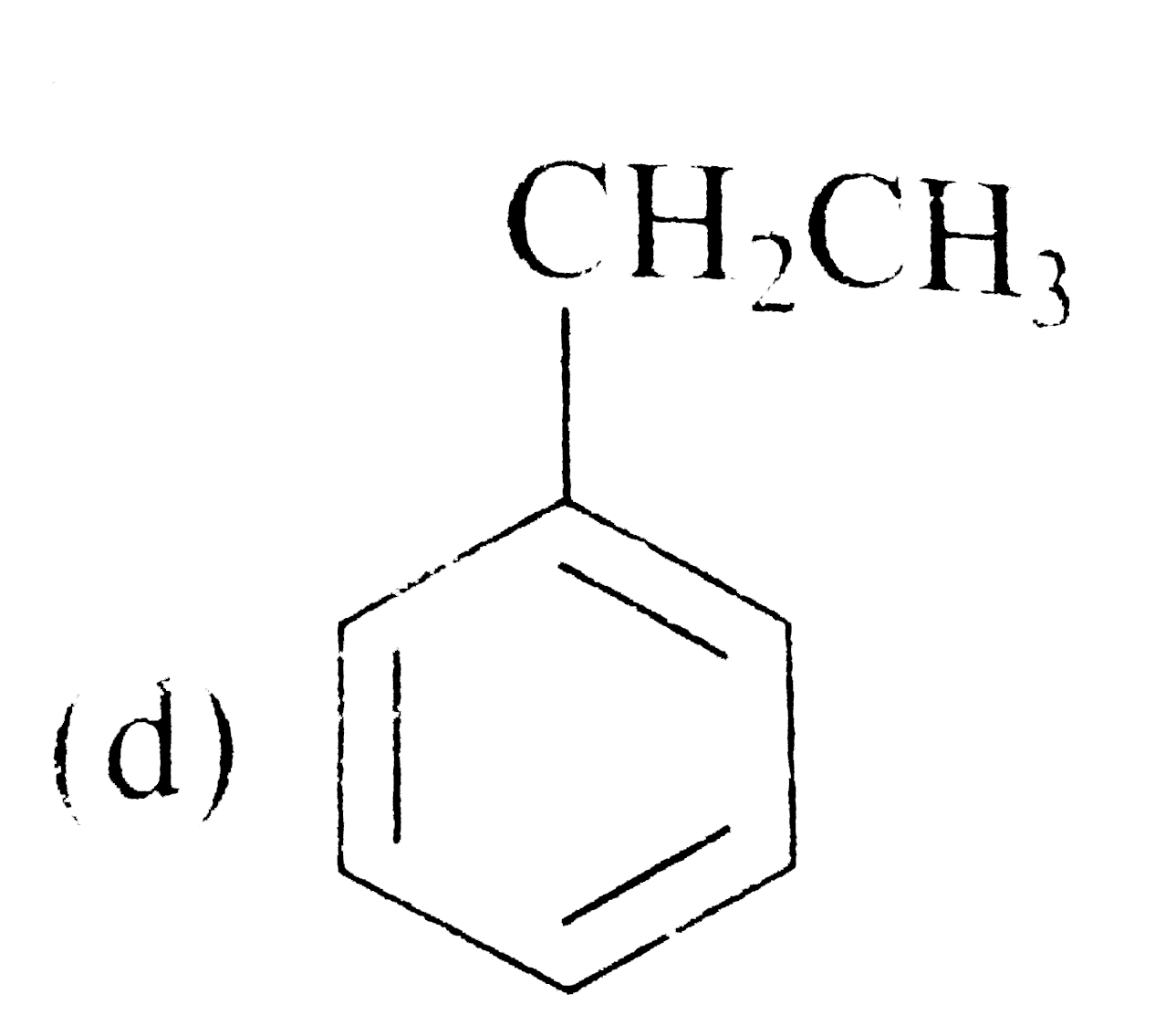
The correct Answer is:A, C, D
a has +M group, c and d have more no.of∝−H
Similar Questions
Which of the following compounds/ion has maximum electron density in benzene ring?
View SolutionIdentification of +m & −m groups : If the first atom of the group has lone pair or negative charge shows +m effect. +m group increases electron density at ortho and para position of benzene ring. If the group has vacant p−orbital on first atom, also a multipal bonded group in which second atom is more electronegative than the first then it shows −m effect. −m group decreases electron density of ortho and para position of benzene ring.
Which of the following compound/s have more electron density than
View Solution
Knowledge Check
Which of the following aromatic ring have greater electron density than ,
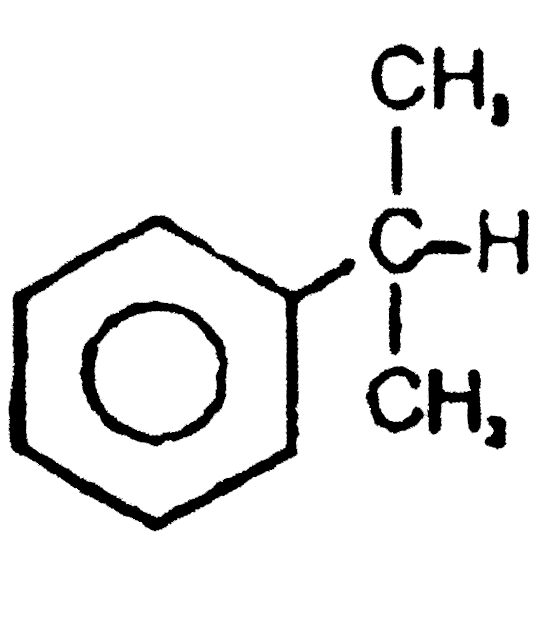 A
A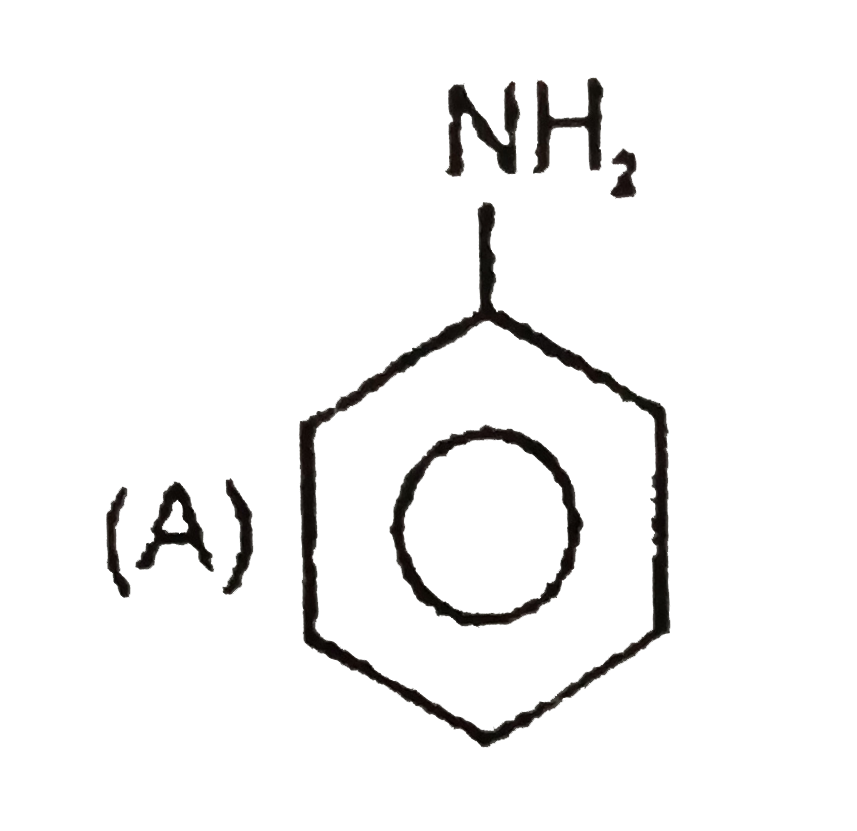 B
B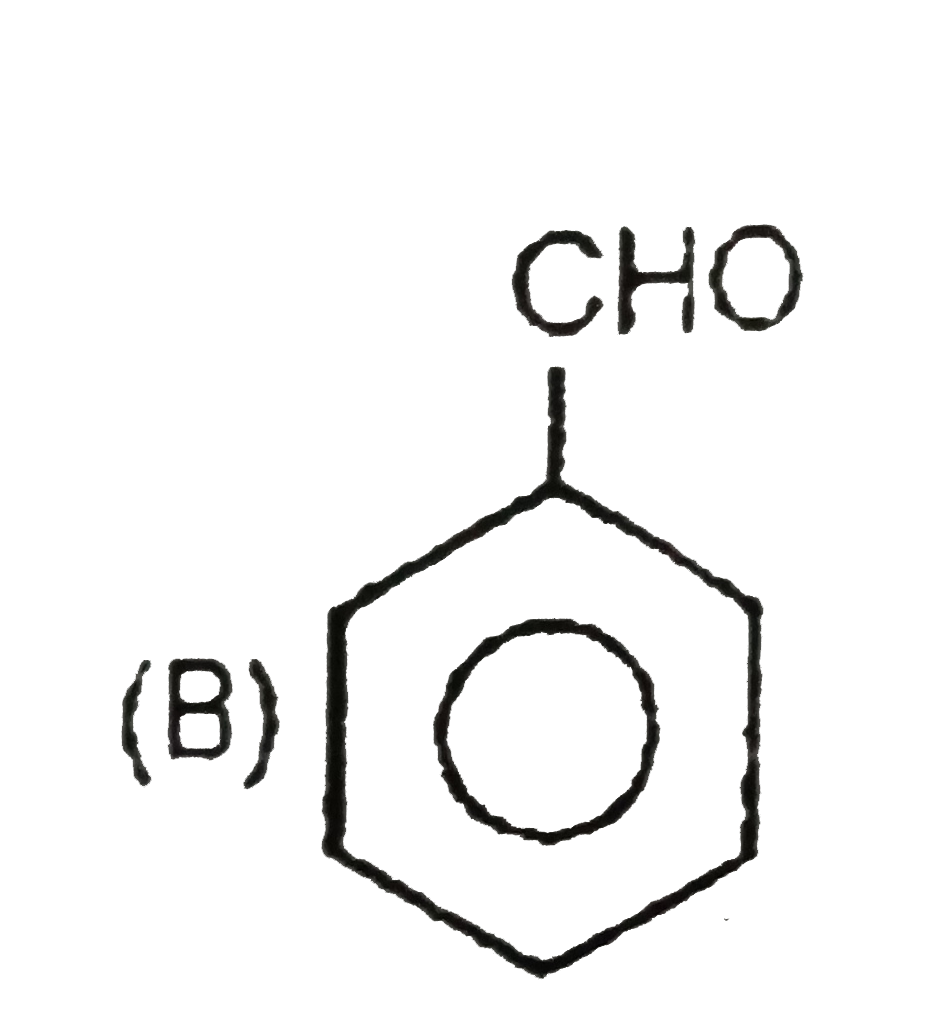 C
C D
D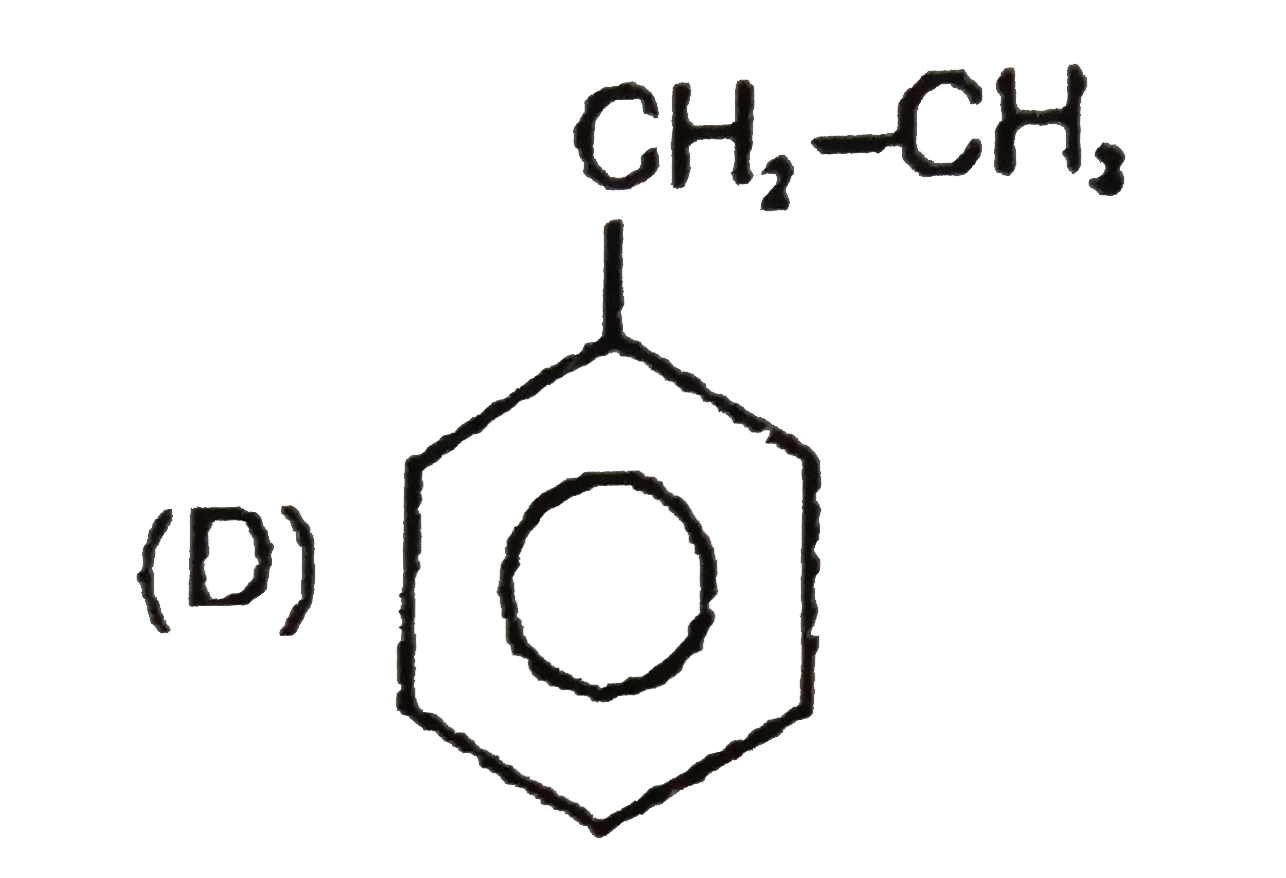
Which of the following benzene ring has greater electron density than Toulene
A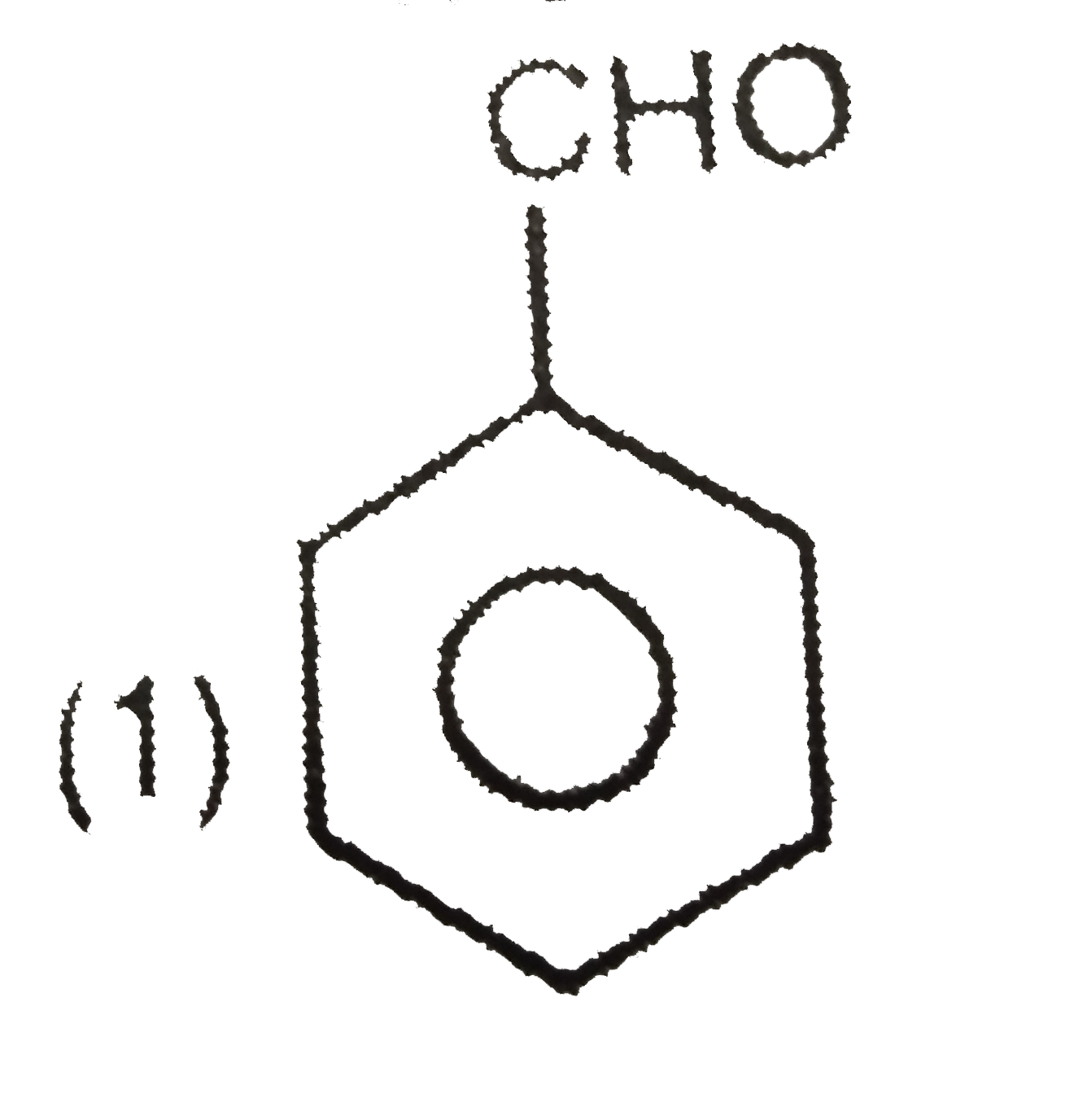 B
B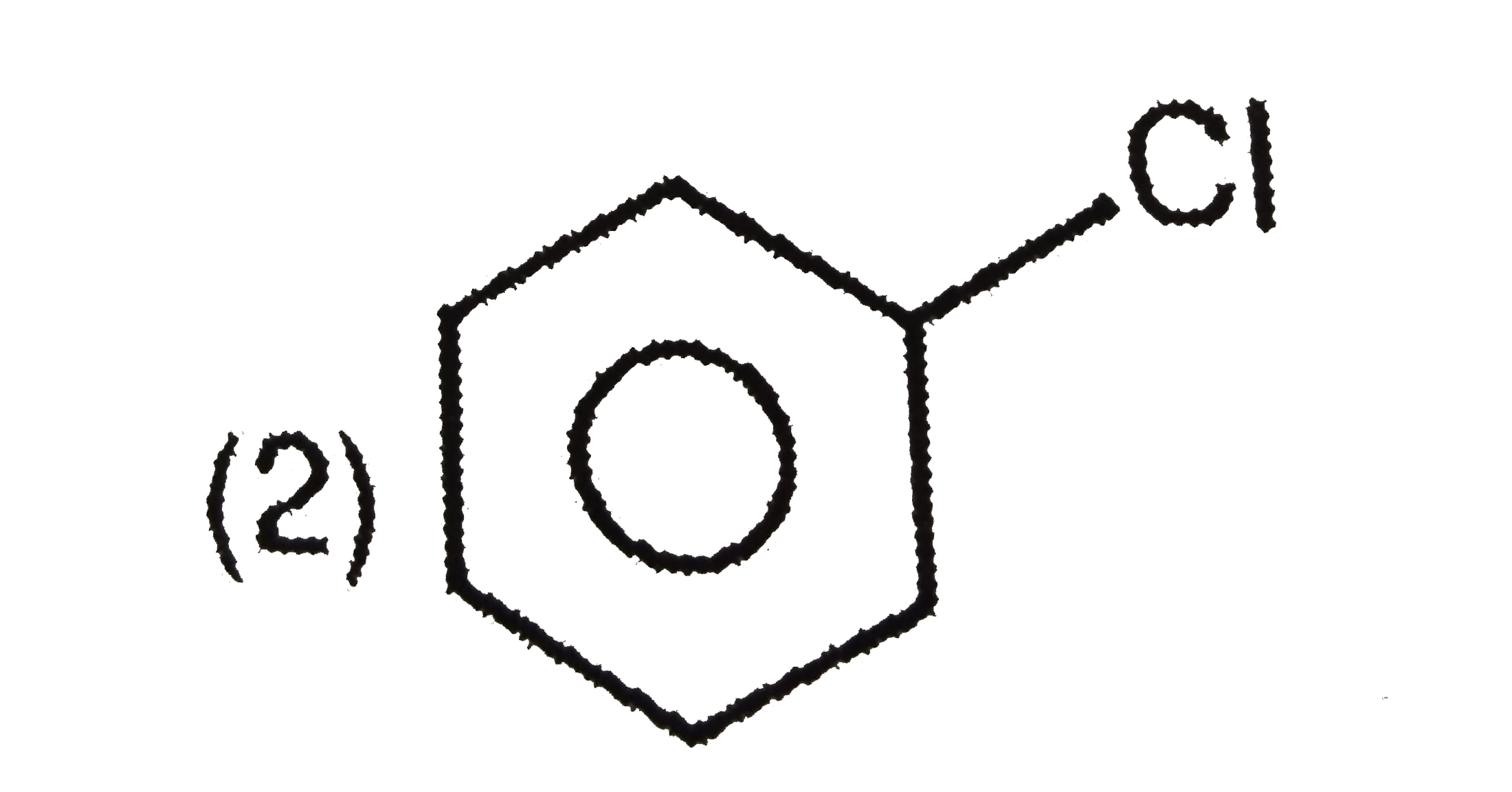 C
C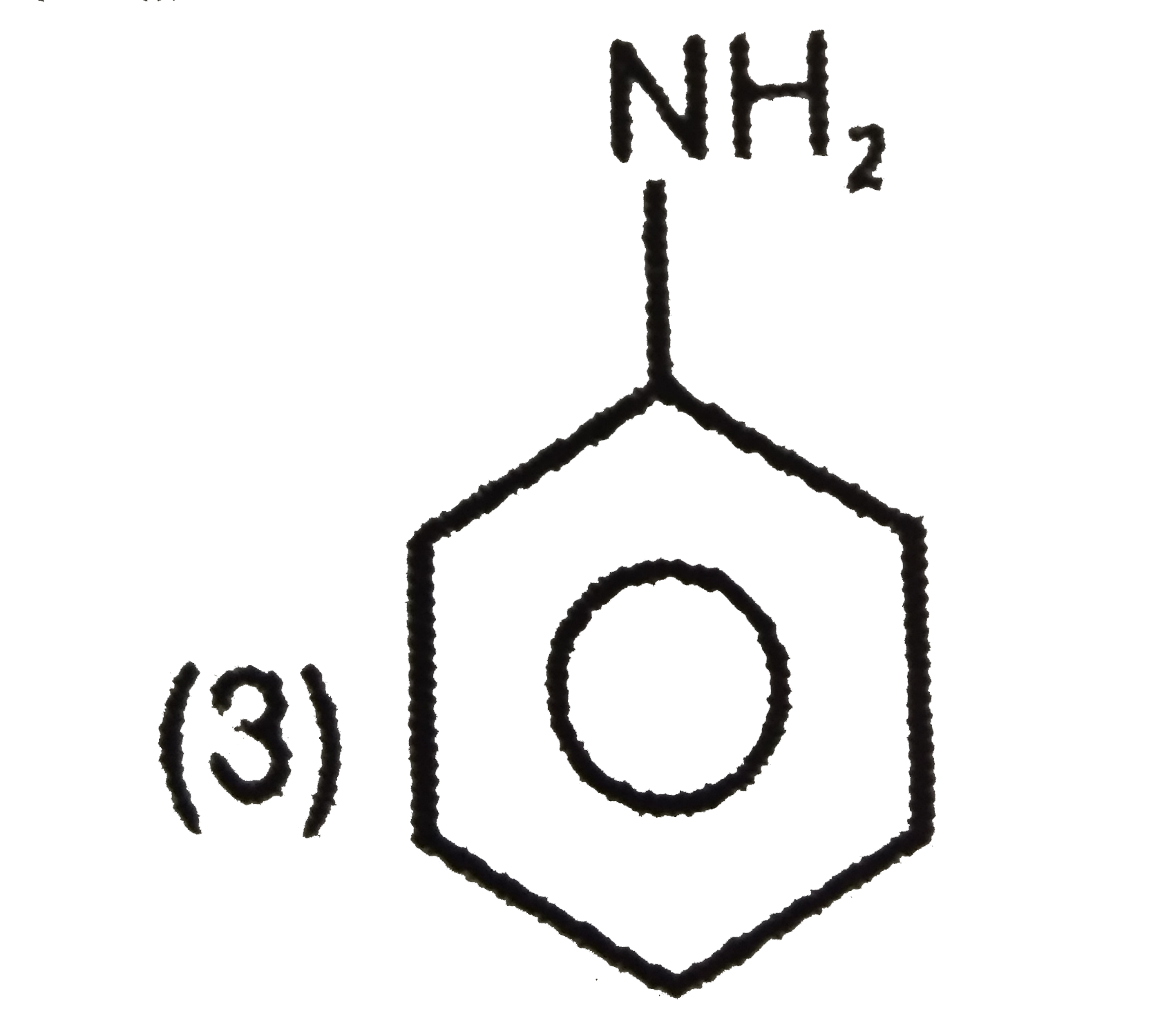 D
D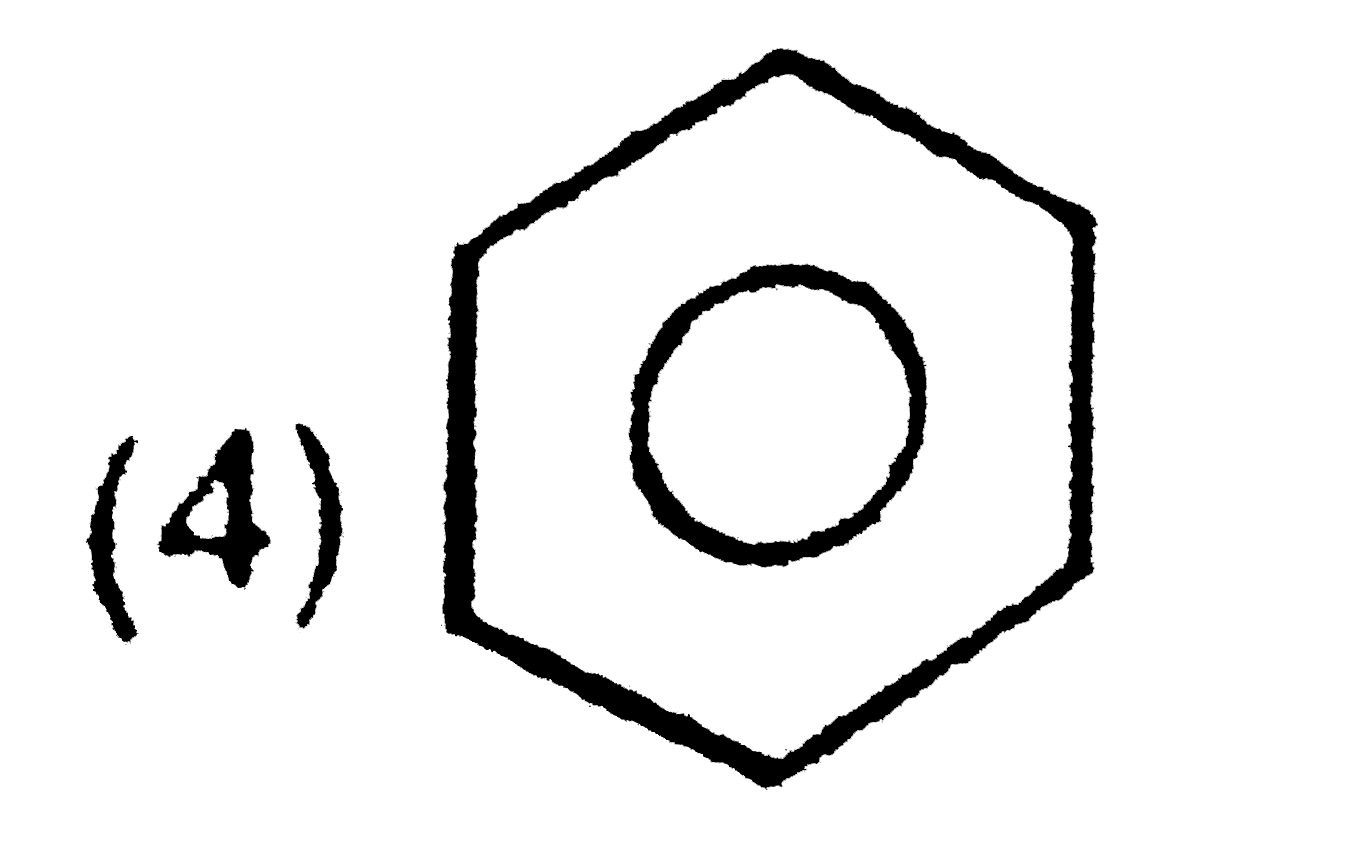
Which of the following compound/s have more electron density than
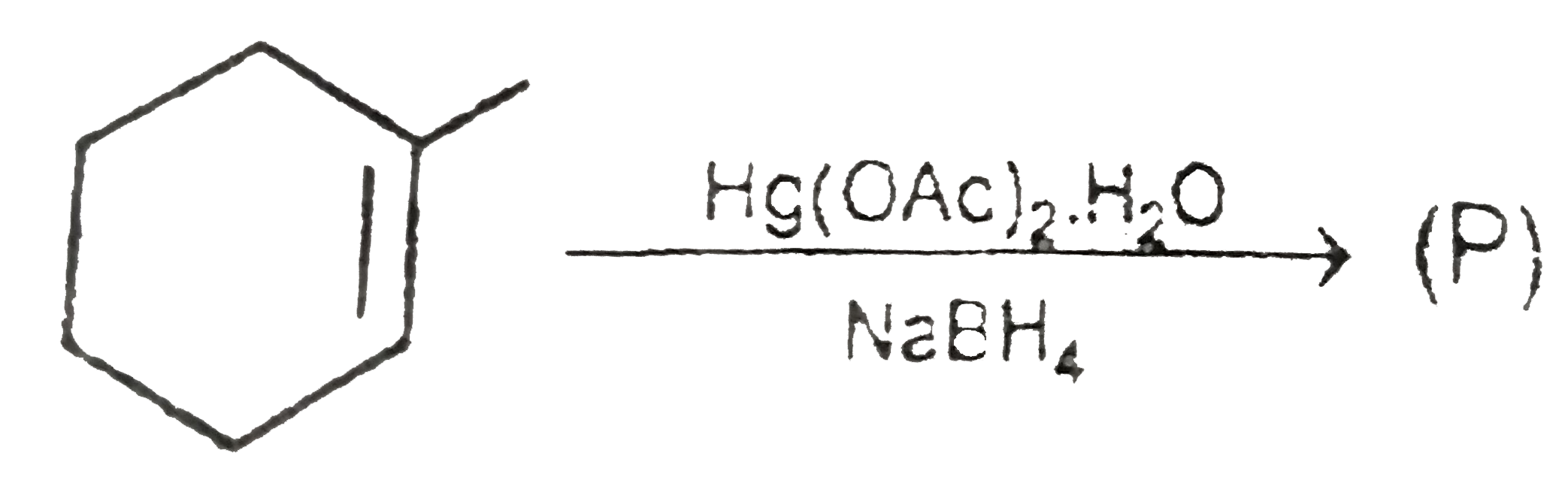 A
A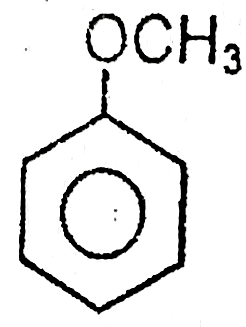 B
B C
C D
D
Which of the following has/have aromatic character:
View SolutionWhich of the following pairs of d-orbitals will hare electron density along the axes ?
View SolutionWhich of the following pairs of d-orbitals will hare electron density along the axes ?
View SolutionWhich of the following pairs of d-orbitals will have electron density along the axes?
View SolutionWhich of the following ions have radius greater than F−?
View Solution
