In a reaction between A and B , the initial rate of reaction (r0) was measured for different initial concentrations of A and B as given below:

What is the order of the reaction with respect to A & B ?
Let the order of the reaction with respect to A be α and with respect to be β.∴Rate,r0=[A]α[B]β
∴(r0)1=5.07×10−5=k(0.2)α(0.3)β ...[1]
(r0)2=5.07×10−5=k(0.2)α(0.1)β ...[2]
(r0)3=5.07×10−5=k(0.2)α(0.1)β ...[3]
∴(r0)1(r0)2=1=[0.30.1]β=3β∴β=0
and (r0)3(r0)2=7.165.07=[0.40.2]α[0.050.1]β
or, 1.412=2α[12]β=2α(∴β=0)
or, log1.412= α2 or, α=0.14980.3010=0.4976≡0.5
∴ Order with respect to A is 0.5 and with respect to B is 0.
Topper's Solved these Questions
- CHEMICAL KINETICSBook:CHHAYA PUBLICATIONChapter:CHEMICAL KINETICSExercise:HIGHER ORDER THINKING SKILL (HOTS) QUESTIONS
Explore 18 Videos
- CHEMICAL KINETICSBook:CHHAYA PUBLICATIONChapter:CHEMICAL KINETICSExercise:ADVANCED LEVEL NUMERICAL BANK
Explore 16 Videos
- CHEMICAL KINETICSBook:CHHAYA PUBLICATIONChapter:CHEMICAL KINETICSExercise:SOLVED NCERT TEXTBOOK PROBLEMS (NCERT INTEXT QUESTIONS)
Explore 21 Videos
- BIOMOLECULESBook:CHHAYA PUBLICATIONChapter:BIOMOLECULESExercise:Practic Set 29
Explore 1 Video
- CHEMICAL THERMODYNAMICSBook:CHHAYA PUBLICATIONChapter:CHEMICAL THERMODYNAMICSExercise:Practice Set 6
Explore 15 Videos
Similar Questions
In a reaction, between A and B, the initial rate of reaction (r0) was measured for different initial concentrations of A and B as given below :

What is the order of reaction with respect to A and B ?
View SolutionIn a reaction between A and B, the initial rate of reaction was measured for different initial concentrations of A and B as given below:
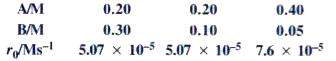
What is the order of reaction with respect to A and B ?
View Solution
Knowledge Check
In a reaction between A and B, the initial rate of reaction r0 was measured for different initial concentrations of A and B as given below :

The order of the reaction with respect to A isA1.5B0.5C1D2In a reaction between A and B, the initial rate of reaction was measured for different initial concentration A and B as:
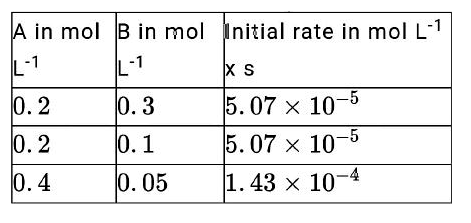
Find the order of reaction with respect to A and B.A1,0B1,1C1,5,0D1,5,1For a reaction between A and B, the initial rate of reaction is measured for various initial concentrations of A and B . The data provided are :
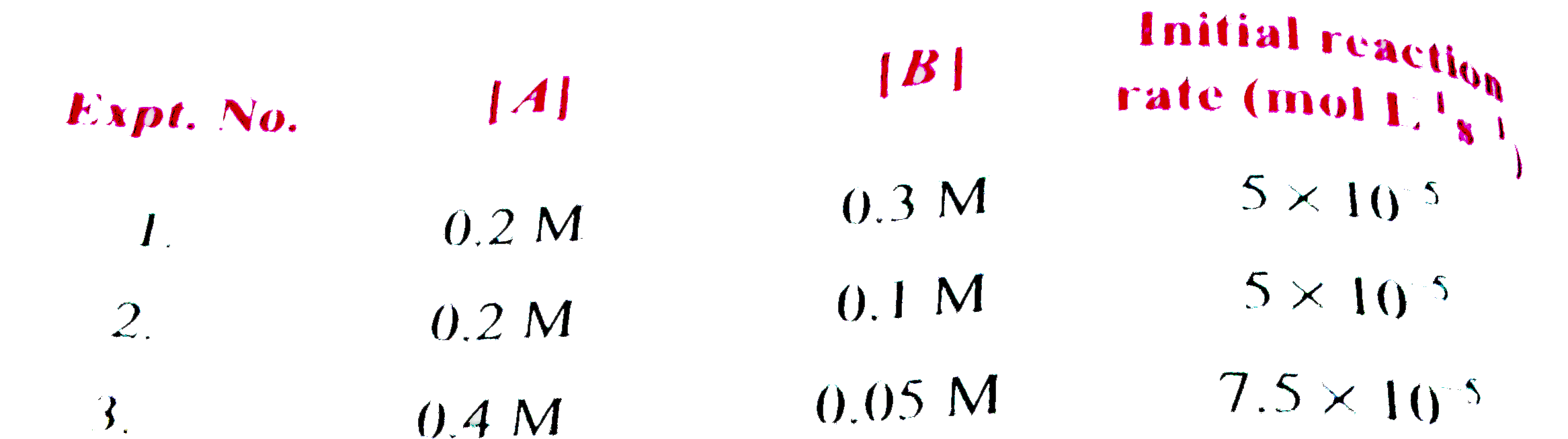
The overall order of the reaction is :Aone(1)Btwo (2)Ctwo and a half (2.5)Dbetween 0 and 1
In a reaction between A and B, the initial rate of reaction was measured for different initial concentrations of A and B as given below:
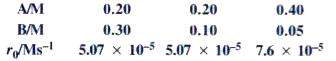
What is the order of reaction with respect to A and B ?
View SolutionIn a reaction between A and B, the initial rate of reaction was measured for different initial concentrations of A and B as given below:
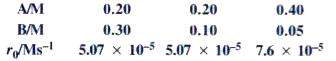
What is the order of reaction with respect to A and B ?
View SolutionIn a reaction between A and B, the initial rate of reaction was measured for different initial concentration of A and B as given ahead:

what is the order of reaction with respect to A and B?
View SolutionConsider the reaction, A+B→C+D.
The initial rate for different initial concentrations of the reactants are given below :
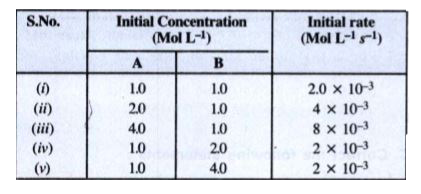
Calculate the rate constant.
View SolutionFor a reaction between A and B, the initial rate of reaction is measured for various initial concentrations of A and B. The data provided are

The overall order of the reaction is
View Solution

