Select reaction in which oxidation number of copper is not changed in final product:
A
CuSO4 solution NaCN(aq)(Excess)−−−−−−−−−−−−→
B
CuSO4 solution KI(Solution)−−−−−−−−→
C
CuSO4 solution S2O2−3(aq)(Excess)−−−−−−−−−−−−→
D
CuSO4 solution KOH(Excess)−−−−−−−−→
Video Solution
Text Solution
The correct Answer is:D
Cu+2+S2O2−3→Cu++S4O2−6
Cu++S2O2−3→Cu2S2O3⏐↓(White ppt) ↓ExcessNa2S2O3[Cu6(S2O3)5]−4(Soluble and colourless)
|
Similar Questions
Knowledge Check
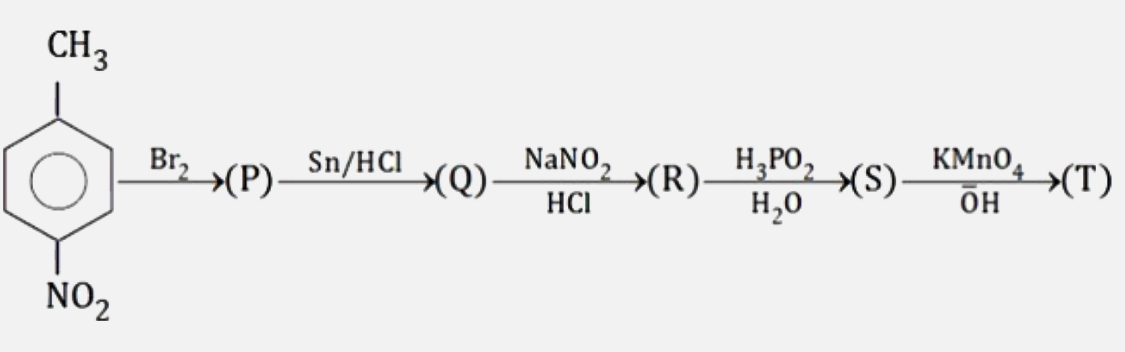
For the reaction
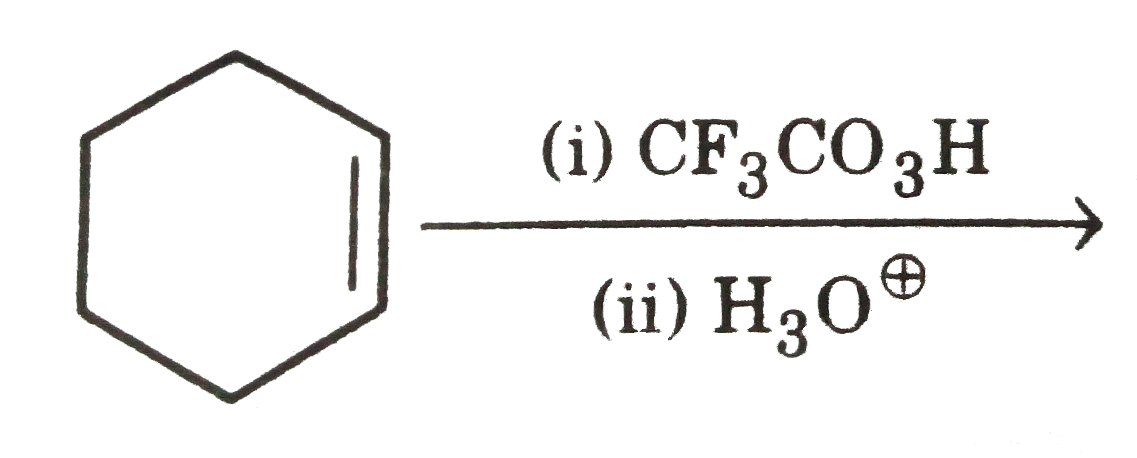 Final product is :A
Final product is :A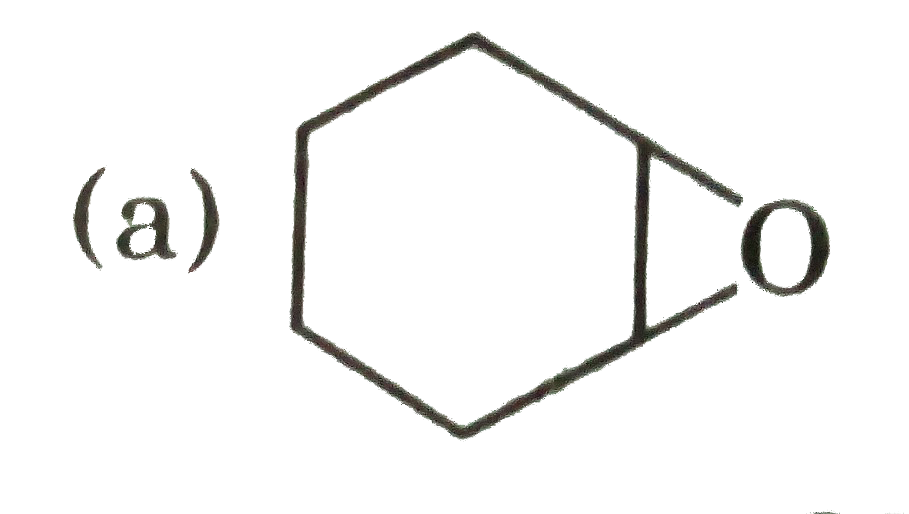 B
B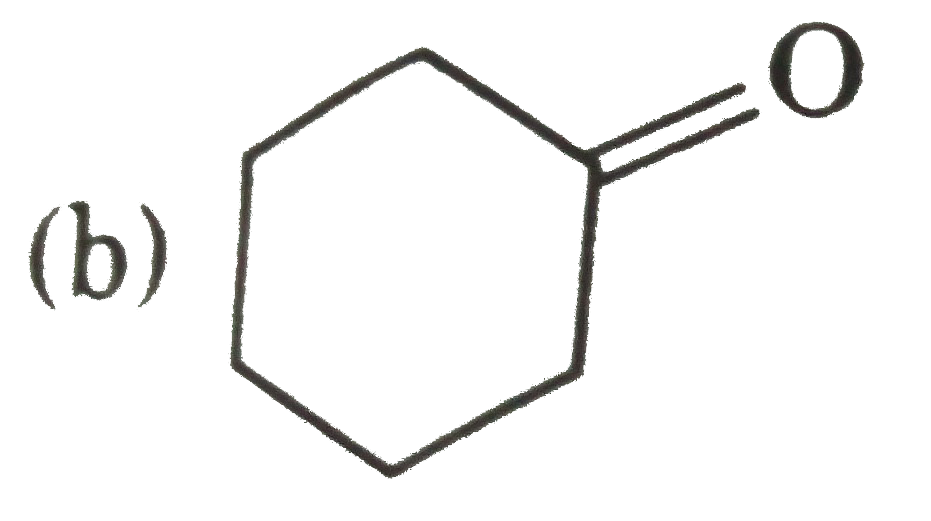 C
C D
D
In which of the following reactions is there a change in the oxidation number of nitrogen atom?
Aheating of ammonium nitrateBHeating of barium nitrateCReaction of Ca3N2 with HClDHeating of ammonium nitriteLead Nitrate is strongly heated. In this reaction the oxidation numbers of following atoms change
AOnly in Pb and NBOnly in N and OCPb, N and ODOnly in N
In which reaction, there is change in oxidation number of N atom?
View SolutionChange in oxidation number is maximum in the following reaction
View SolutionIn the following reaction, final product is :

View SolutionIn the sequence of reactions
_E01_554_Q01.png)
the final product (B) is
View SolutionIn which of the following reactions is there a change in the oxidation number of nitrogen atom?
View Solution