Arrange the following in decresing order of -I effect −C6H5,−NO2,−COOH,−CN,−I,−F,−CH3,−C2H5
−NO2>−CN>−COOH>−F>−I>−CH3>−C2H5.
Similar Questions
Arrange the following:
(i) in decreasing order of pKa values C2H5NH2,C6H5NHCH3,(C2H5)2NHandC6H5NH2.
(ii) in increasing order of basic strength C6H5NH2,C6H5N(CH3)2,(C2H5)2NHandCH3NH2.
View SolutionArrange the following:
(i) In decreasing order of the pKb values:
C2H5NH2,C6H5NHCH3,(C2H5)2NHandC6H5NH2
(ii) In increasing order of basic strength:
C6H5NH2,C6H5N(CH3)2,(C2H5)2NHandCH3NH2
(iii) In increasing order of basic strength:
(a) Aniline, p-nitroaniline and p-toluidine
(b) C6H5NH2,C6H5NHCH3,C6H5CH2NH2
(iv) In decreasing order of basic strength in gas phase:
C2H5NH2,(C2H5)2NH,(C2H5)3NandNH3
(v) In increasing order of boiling point:
C2H5OH,(CH3)2NH,C2H5NH2
(vi) In increasing order of solubility in water:
C6H5NH2,(C2H5)2NH,C2H5NH2.
View SolutionArrange the following in incresing order of basic strength :
C6H5NH2,C2H5NHCH3,C6H5N(CH3)2.
View SolutionArrange the following in incresing order of basic strength :
C6H5NH2,C2H5NHCH3,C6H5N(CH3)2.
View Solution(i) Arrange the following compounds in an increasing order of basic strength :
C6H5NH2,C6H5N(CN3)2,(C2H5)2NHandCH3NH2
(ii) Arrange the following compounds in a decreasing order of pKb values:
C2H5NH2,C6H5NHCH3,(C2H5)2NHandC6H5NH2
View Solution(i) Arrange the following compounds in an increasing order of basic strength :
C6H5NH2,C6H5N(CN3)2,(C2H5)2NHandCH3NH2
(ii) Arrange the following compounds in a decreasing order of pKb values:
C2H5NH2,C6H5NHCH3,(C2H5)2NHandC6H5NH2
View SolutionArrange the following in increasing order basic strength :
(i) C6H5−NH2,C6H5CH2−NH2,C6H5NH−CH3
(ii)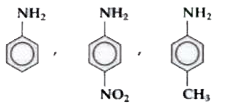
View SolutionArrange the following in increasing order of their basic strength :
(i)C2H5NH2,C6H5NH2,C6H5CH2NH2 and (C2H5)2NH
(ii) C2H5NH2,(C2H5)2NH,(C2H5)3N,C6H5NH2
(iii) CH3NH2,(CH3)2NH,(CH3)3N,C6H5NH2,C6H5CH2NH2
View SolutionArrange the following in increasing order of their basic strength :
(i)C2H5NH2,C6H5NH2,C6H5CH2NH2 and (C2H5)2NH
(ii) C2H5NH2,(C2H5)2NH,(C2H5)3N,C6H5NH2
(iii) CH3NH2,(CH3)2NH,(CH3)3N,C6H5NH2,C6H5CH2NH2
View SolutionArrange the following in decreasing order of solubility in water
(i) CH3CH2OH (ii) CH3OH (iii) C6H5CH2OH.
View Solution

