What is the effect of introducing an alkyl group on the stability of carbocation?
Text Solution
It increases because of hyper conjugation effect.
|
Similar Questions
Knowledge Check
Compare the stability of carbocation
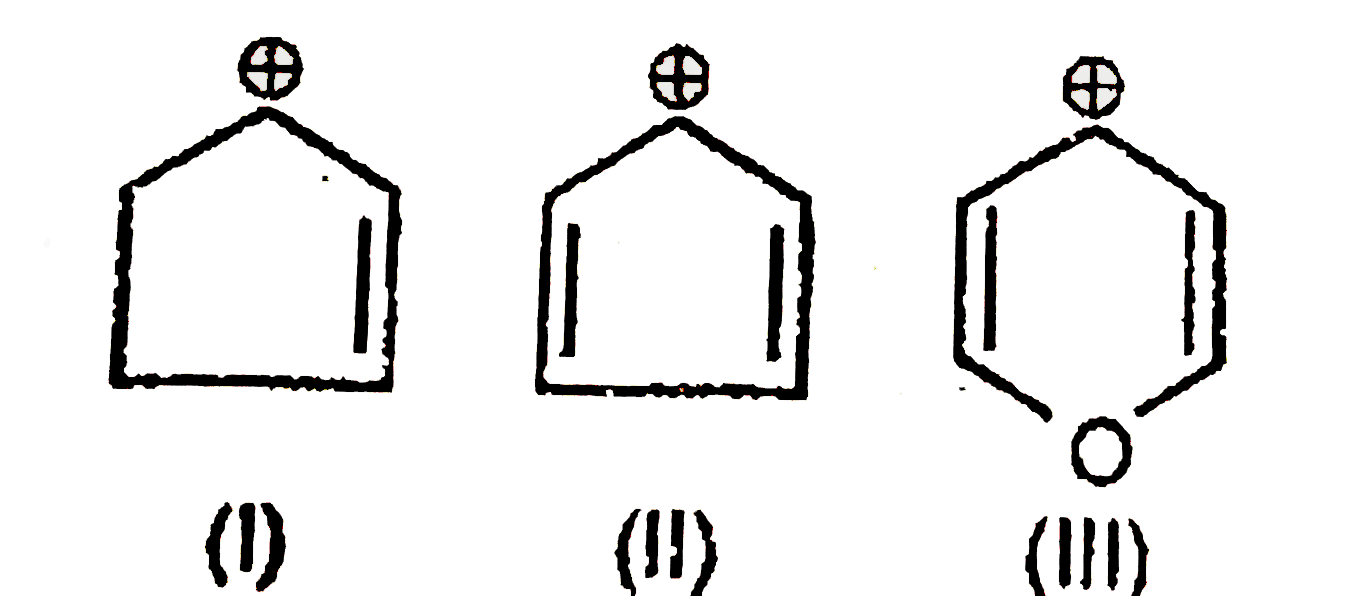 AIII>II>IBII>III>ICIII>I>IIDII>I>III
AIII>II>IBII>III>ICIII>I>IIDII>I>IIIThe stability order for carbocation is :
A2∘>3∘>I∘B3∘>2∘>I∘C3∘>1∘>2∘D1∘>3∘>2∘Compare the stability of following carbocation
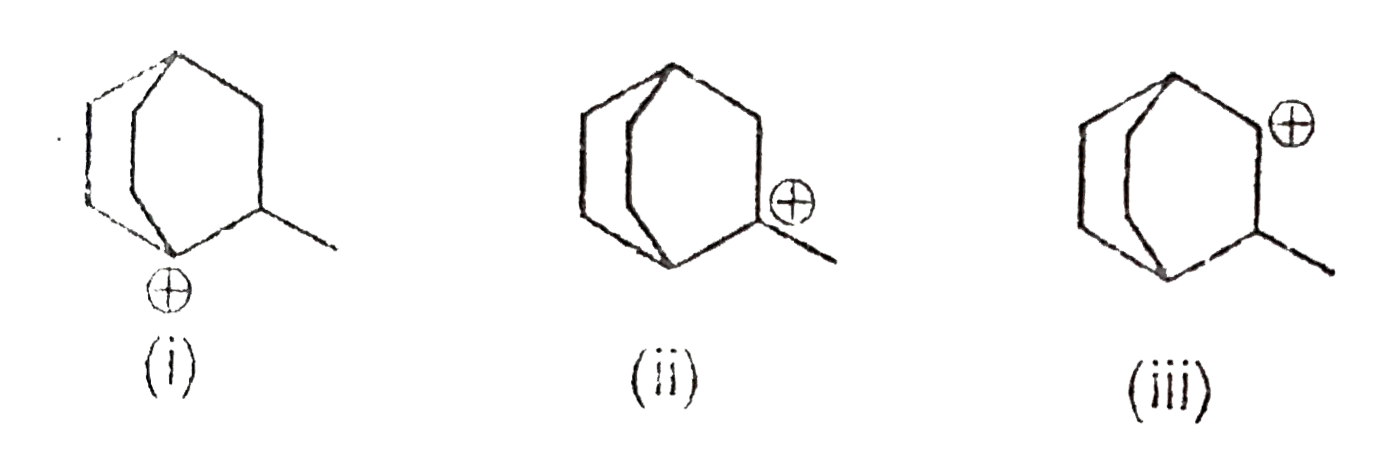 Aii>iii>iBi>iii>iiCii>i>iiiDi>ii>iii
Aii>iii>iBi>iii>iiCii>i>iiiDi>ii>iii
What is the Stability order of carbocation
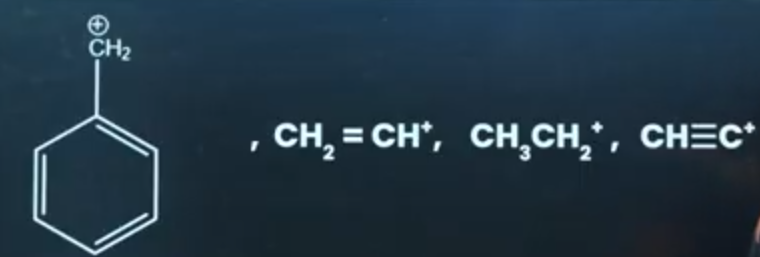
View Solutionstability of carbocations depends on
View SolutionCompare the stability of following carbocations .
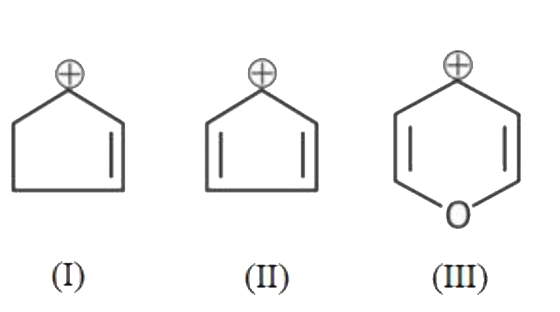
View Solution(A) Tertiary carbocations are generally formed more easily than primary carbocations.
(R) Hyperconjugation as well as inductive effect due to additional alkyl groups stabilize tertiary cabocations.
View SolutionAssertion : Tertiary carbocations are generally formed more easily than primary carbocations.
Reason : Hyperconjugation as well as inductive effect due to additional alkyl groups stabilize tertiary carbocations.
View Solution

